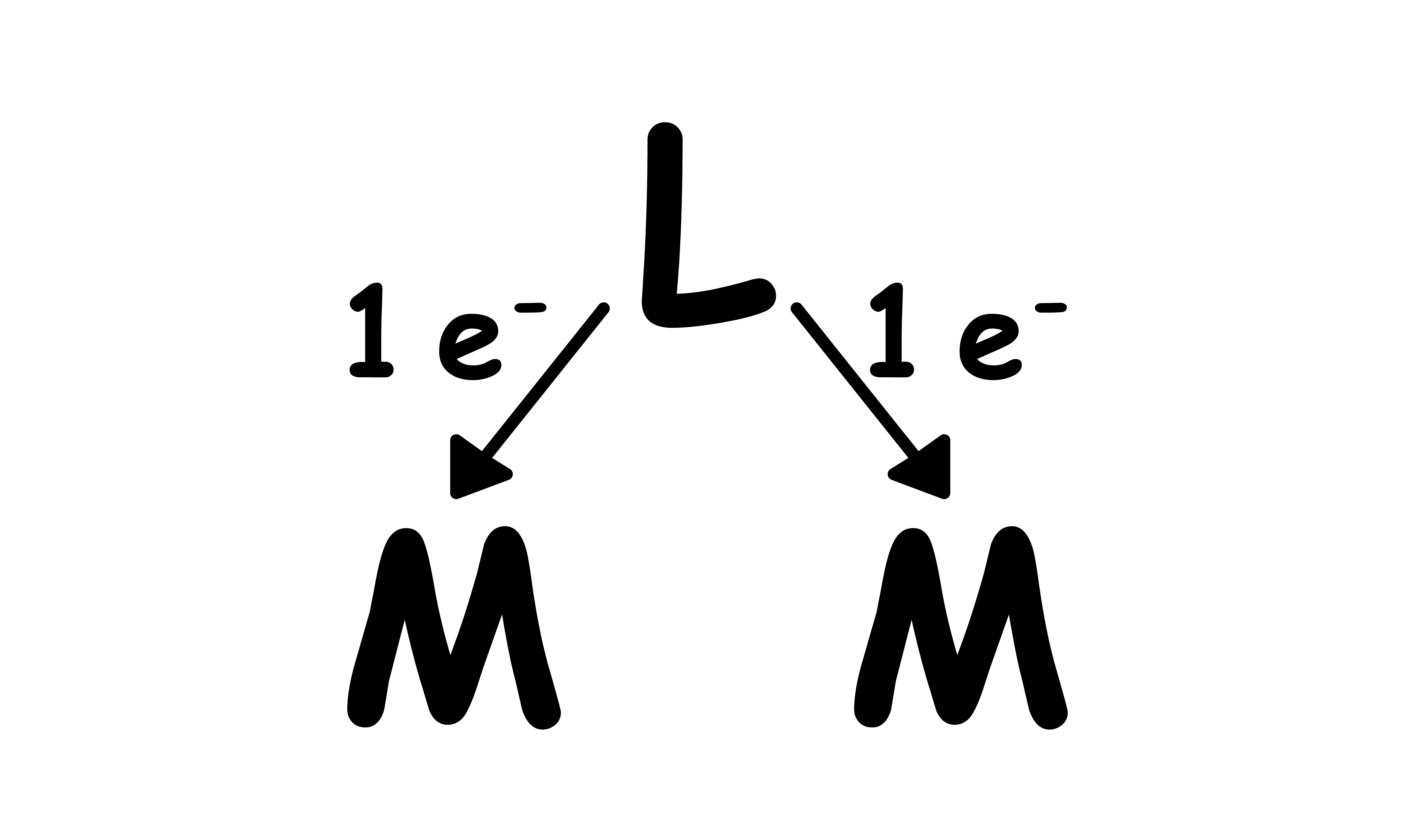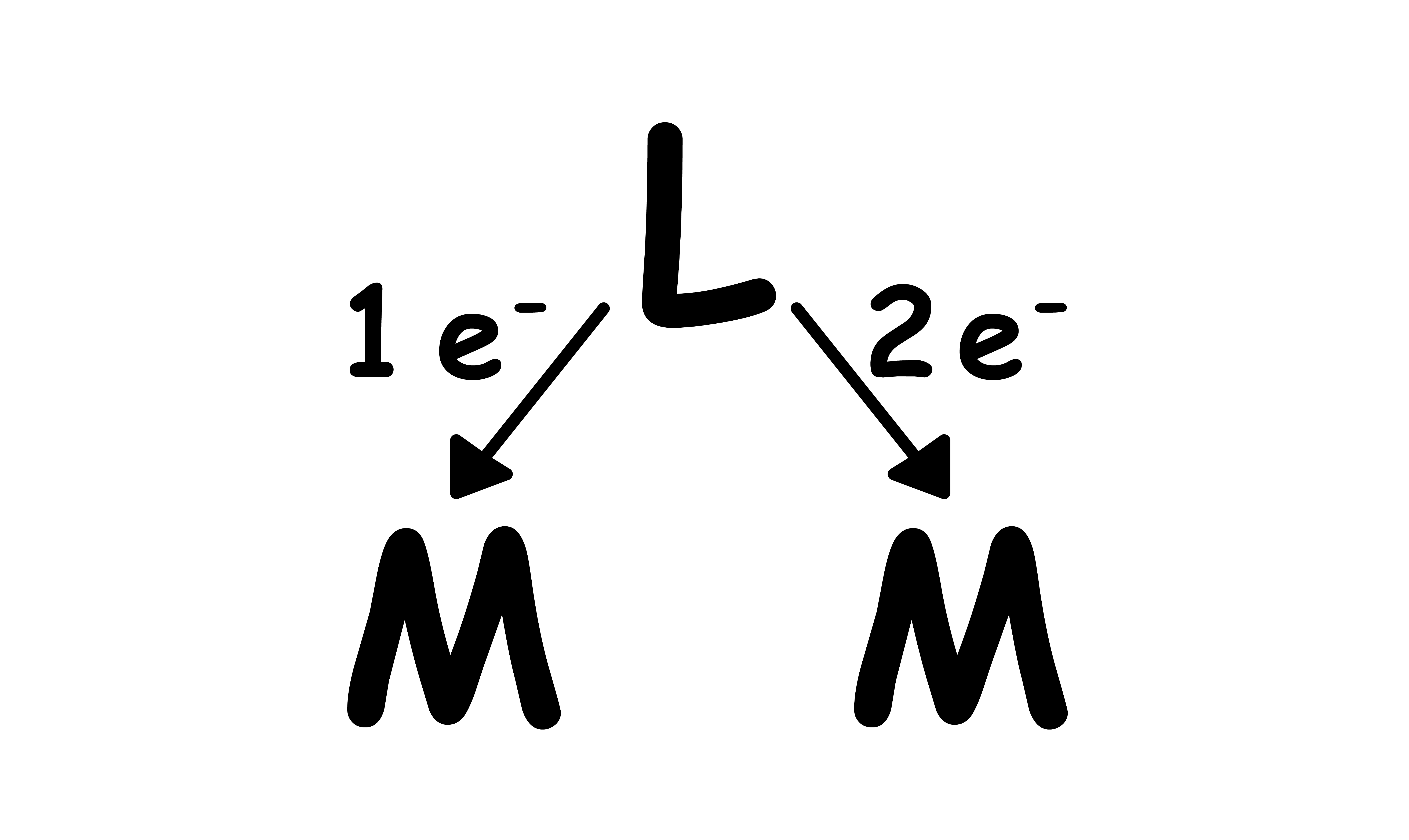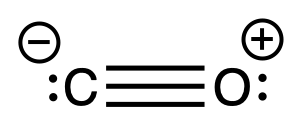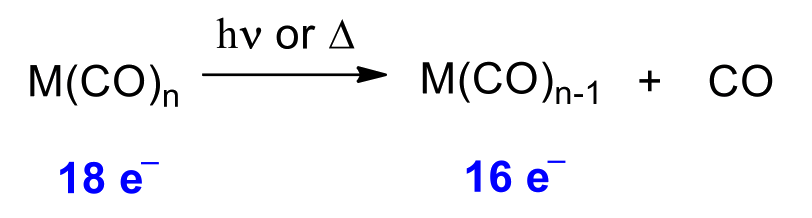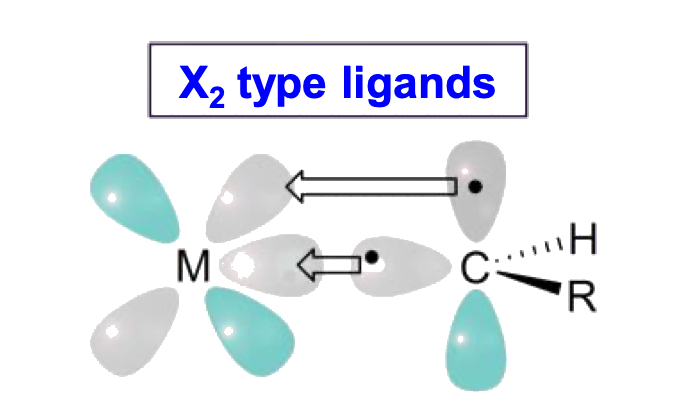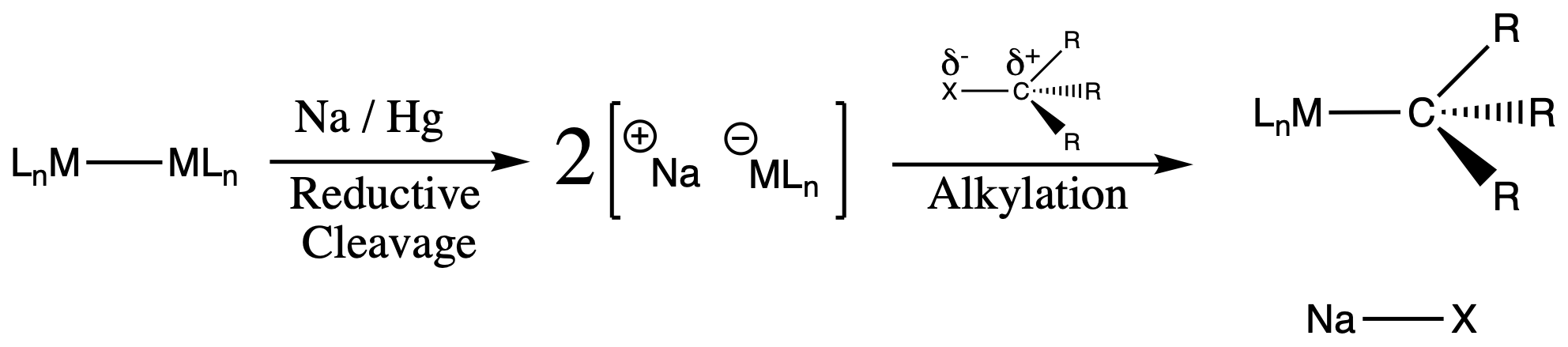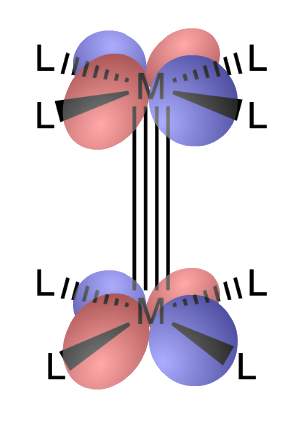Transition metals are defined as partially filled d subshell in the neutral atom or ions
- Group 12 elements have a completely filled d subshell, so they are not considered as transition metals
¶ Important Recap
Feel free to familiarize yourself with year 1 contents here:
Ionization Energy:
- decreases down the group; increases left to right
Metallic radii:
- increases down the group
- decreases left to right
Contractions (experienced by TM & lanthanide):
- increasing Zeff across the period since shielding of increasing electrons are not so effective
High vs low spin
- When one or a whole set of degenerate orbitals are half-filled ( all the electrons ) are unpaired, the next electron can be either added as a spin-pair in the same level or be added as an unpaired electron in the next highest orbital
- High spin complexes are those that possesses a greater number of unpaired electrons, while low spin complexes are those that possesses a smaller number of unpaired electrons
Whether a complex adopts high or spin configuration depends on (crystal field splitting energy) and (e- pairing energy)
- decreases down a group ( since d orbitals become larger and electrons repel each other less ) , while increases down a group, the combined effect is that complexes with metal center at 4d or 5d are usually in low spin
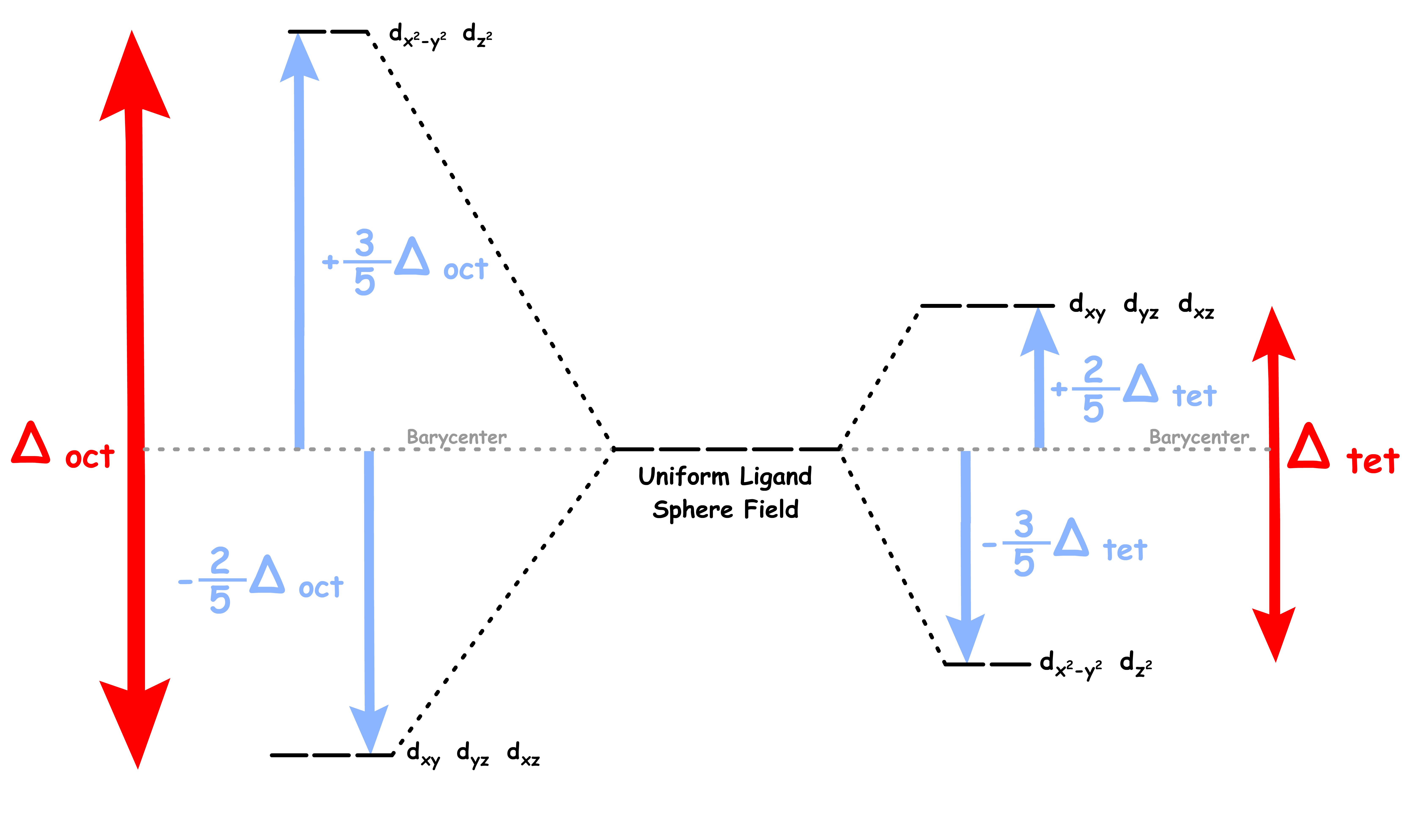
Crystal field splitting (Oh vs Td):
- The crystal field splitting energy of octahedral complexes, , is typically larger than that of tetrahedral complexes
Ionic radii
- Factors influencing radii are: spin, charge, electronic configuration
- Low spin complexes have a smaller radii, electrons are in interaxial d orbtials i.e. less electron repulsion
- High spin complexes have a larger radii, electrons are in axial d orbitals that point towards the ligands
¶ Factors affecting
The crystal field splitting energy is denoted as Δgeometry
Δ increases with increasing oxidation state of the metal center
- As we increase the oxidation state of the metal, the center becomes more positively charged and the ligands are pulled in closer
- The close proximity allows for a stronger interaction between the d-orbitals and the ligands
Δ increases down the group of transition metal in the Periodic Table
- As we go down the group, the d-orbitals of the transition metal becomes larger
- The large d-orbitals can extent further, thus allowing better interactions with the ligands
- Note that the pairing energy also decreases down the group due to a smaller electronic repulsion in a larger orbital
- These two effects causes low spin to be more favorable for metal complexes in the lower rows
Spectrochemical Series
- Ligands that produce a large splitting are called strong field ligands, and those that produce a small splitting are called weak field ligands
¶ Ligand field theory
Crystal field only assumes electrostatic interactions & point charges, which cannot explain the spectrochemical series. Ligand field theory is here to help.
- We need to recall the MO description of Oh complexes (term 1 Dr Kafizas), there are two scenarios
- We will mainly focus on octahedral complexes
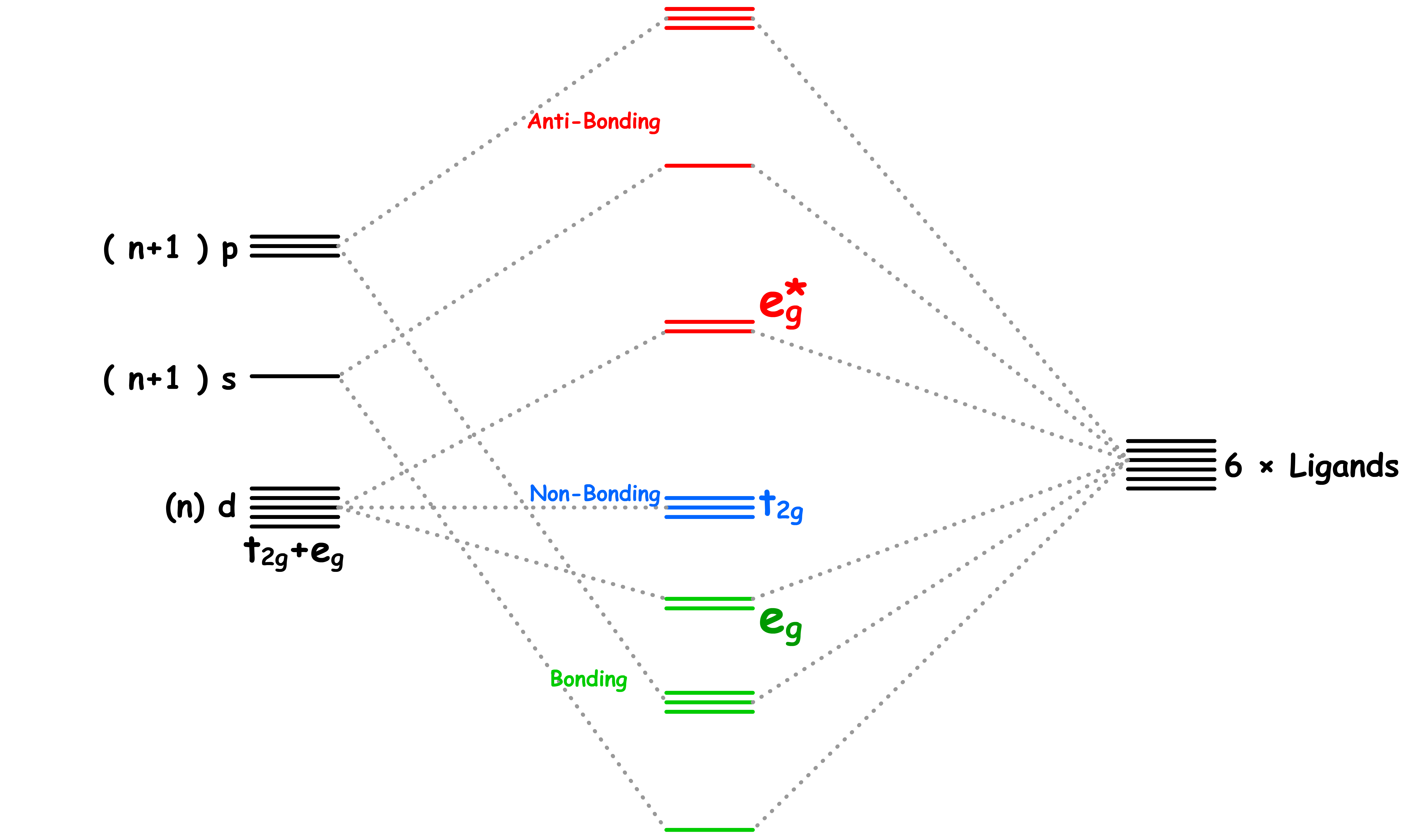
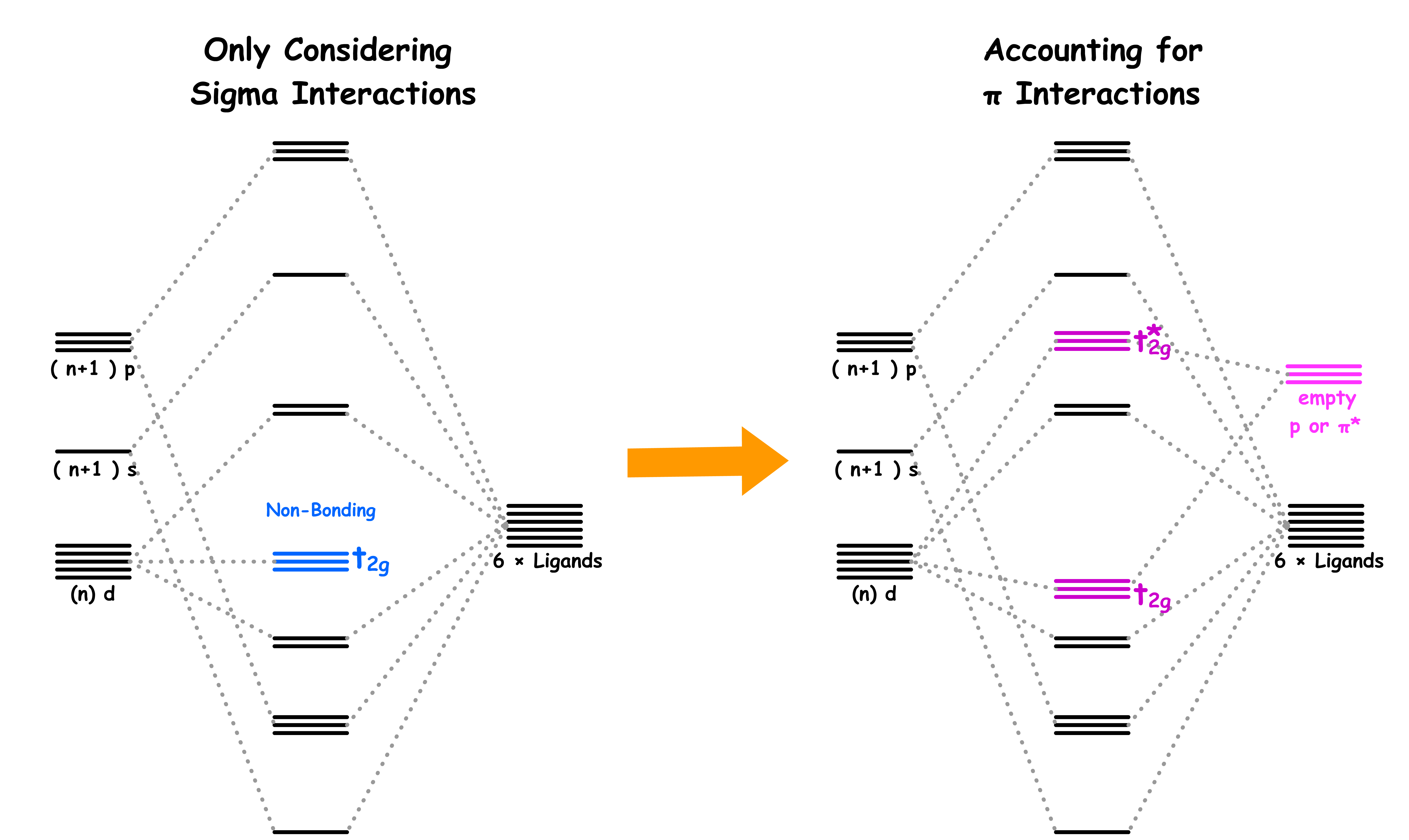
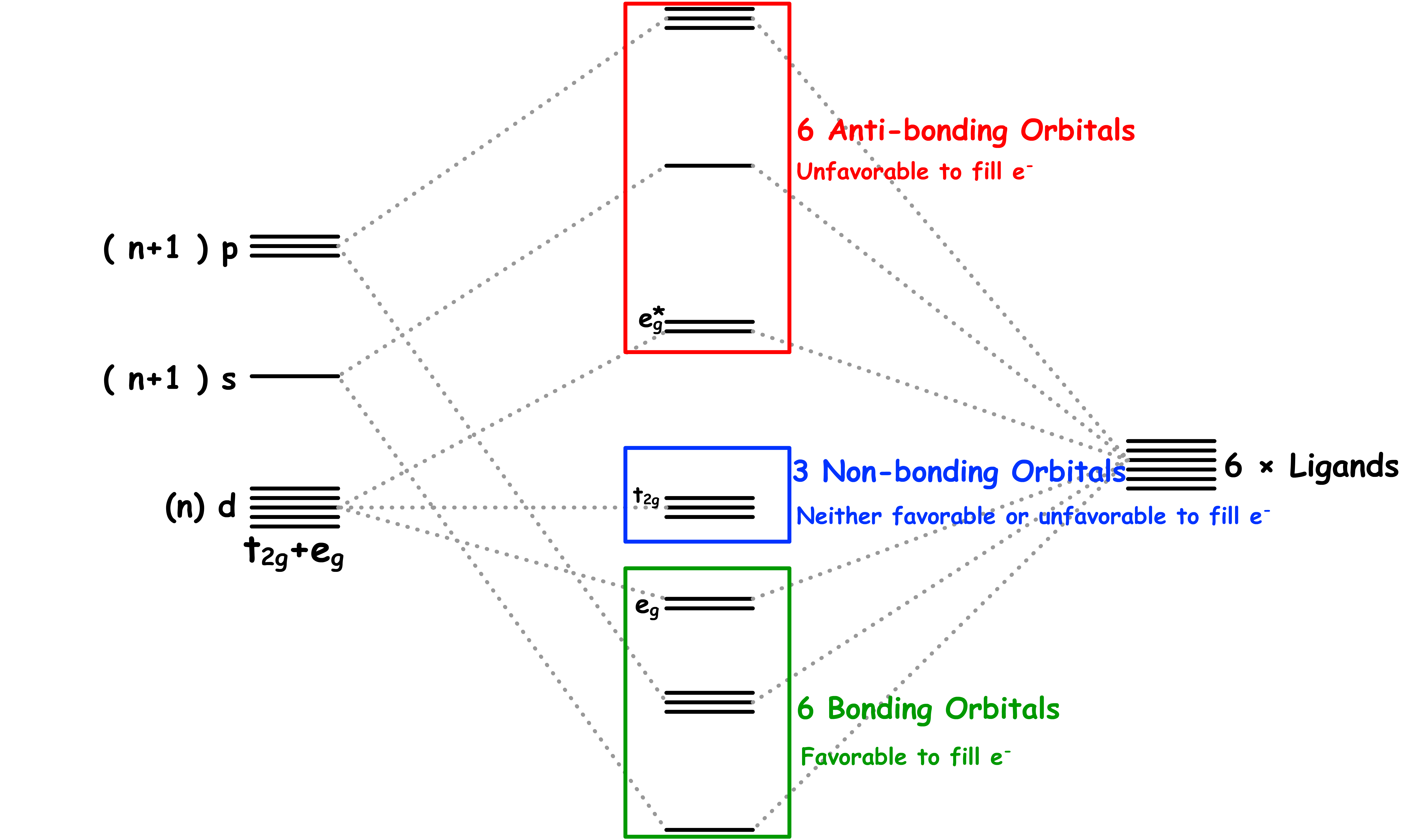
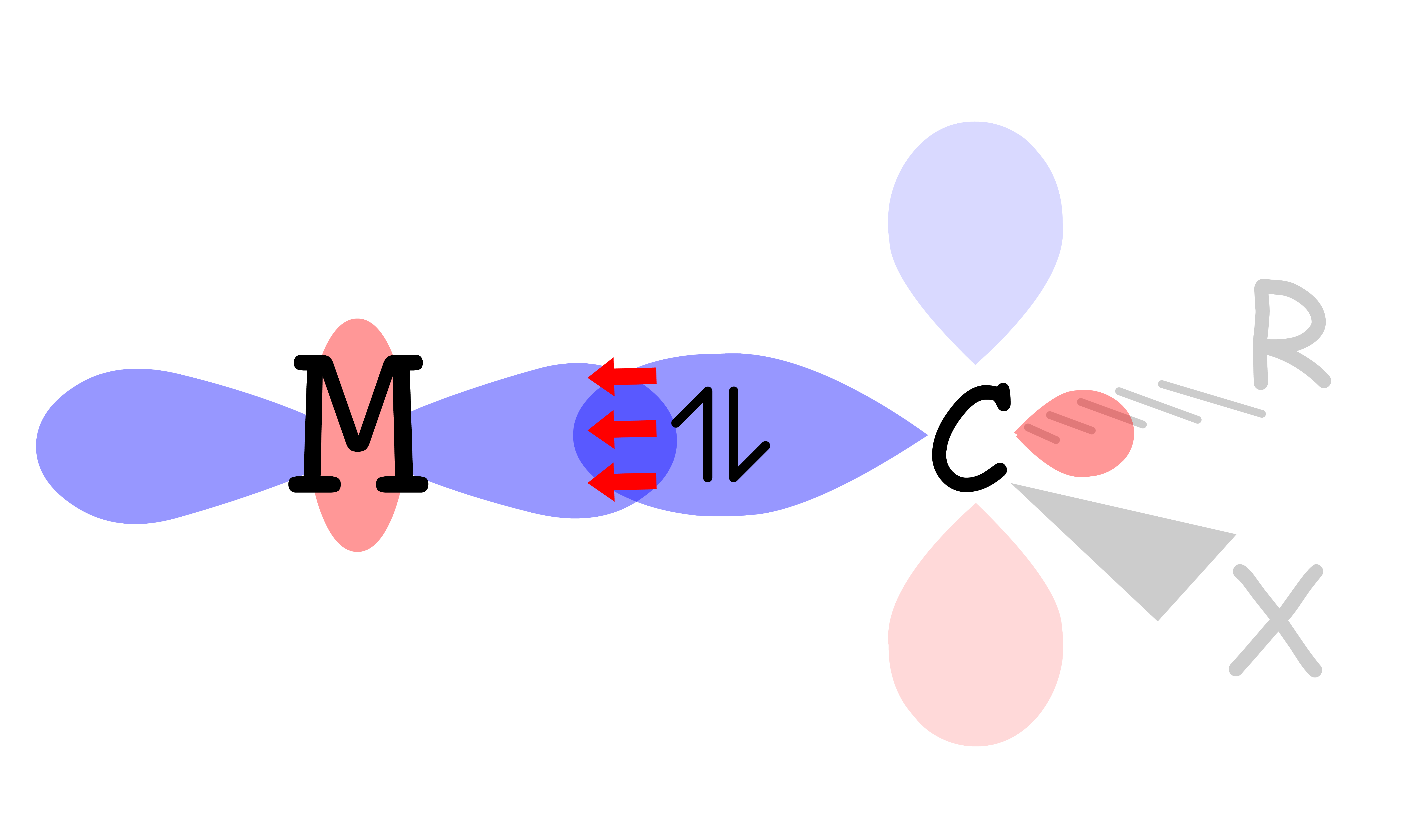
¶ σ-Interactions in ML6 Complexes
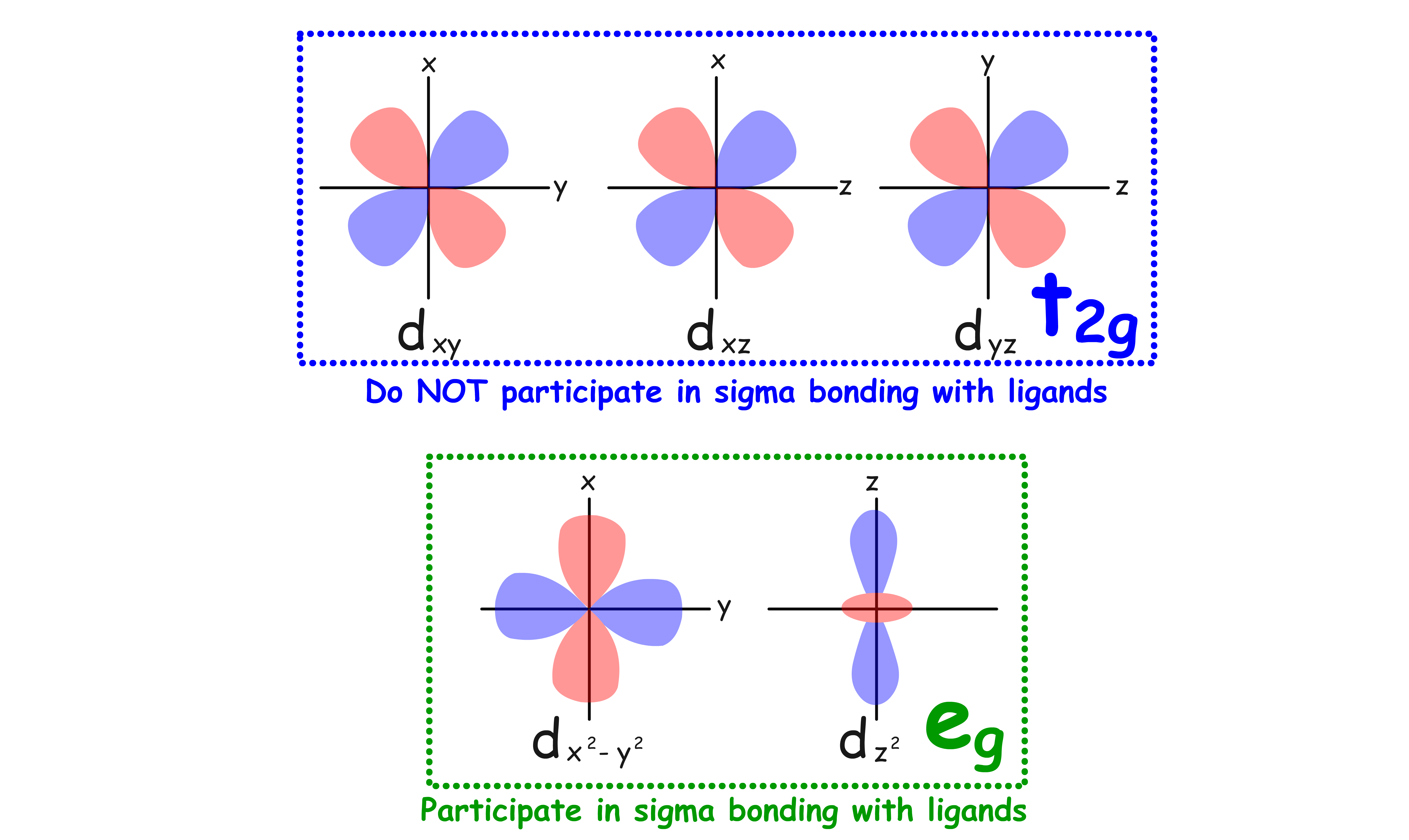
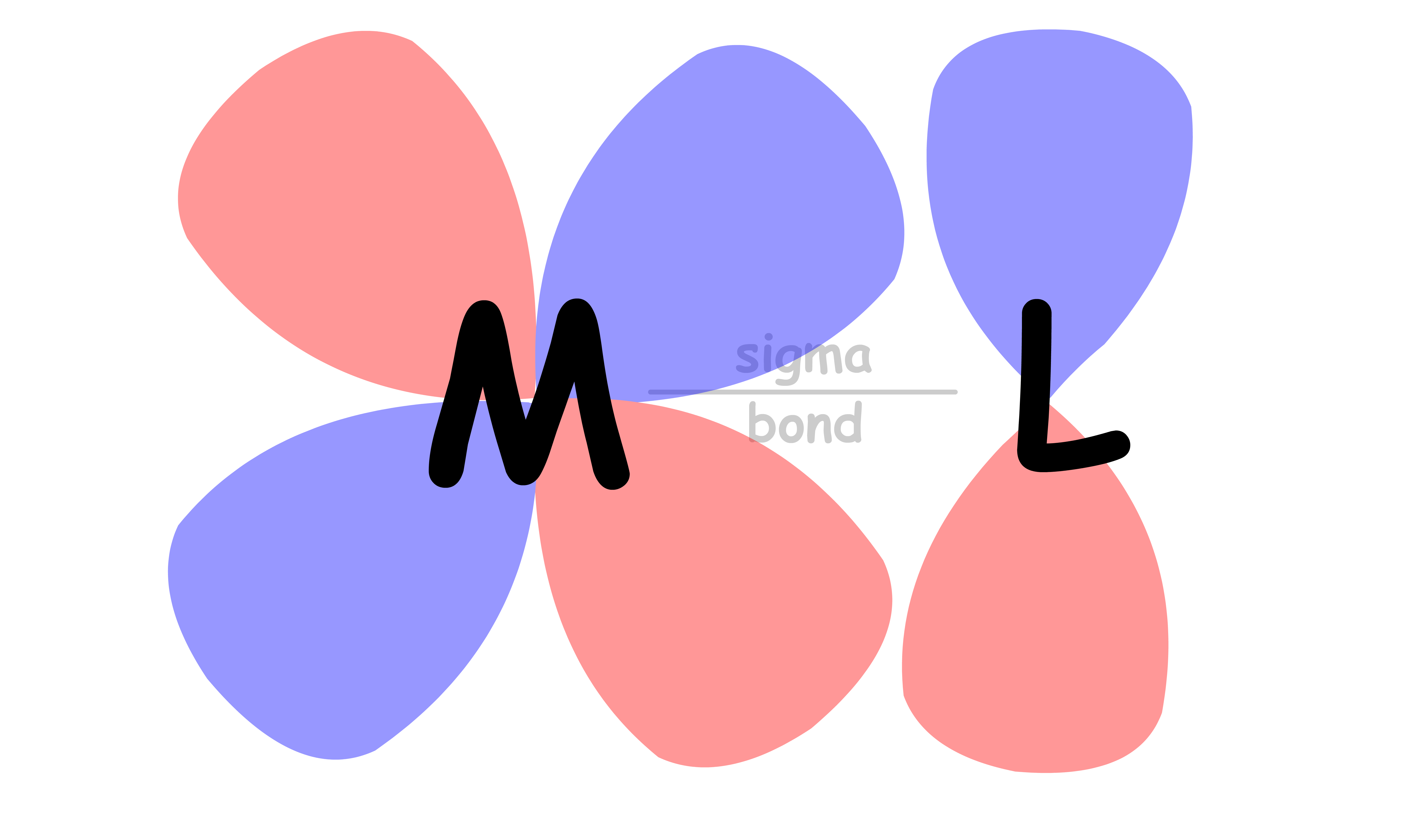
All ligands are bonded to the metal center via sigma bonding
- There are two sets of d-orbitals, the set and the set. The orbitals participate in sigma bonding with the ligands while the orbitals do not
- The reason why only the orbitals participate in sigma bonding is because the ligands are located on the Cartesian axes in an octahedral complex
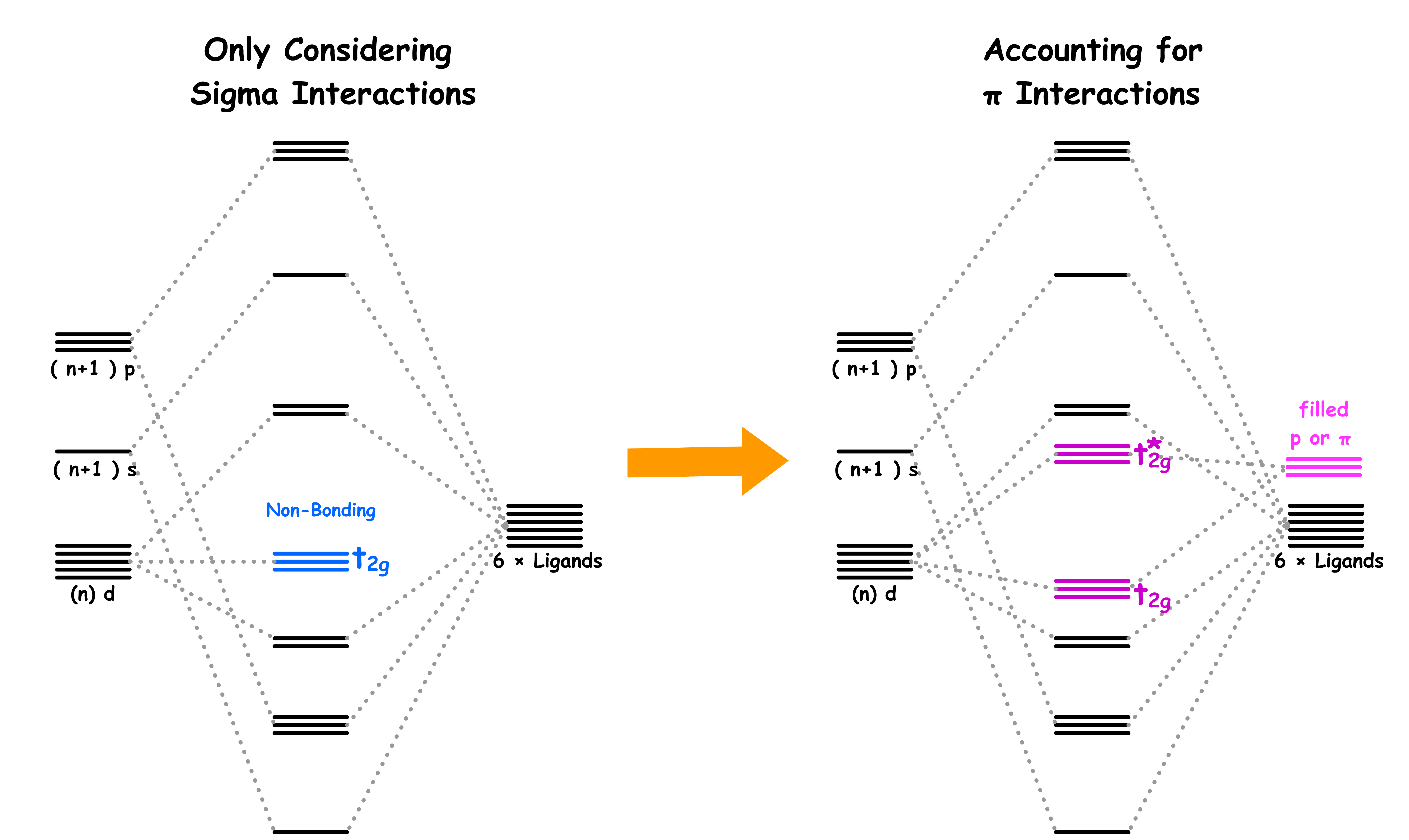
- Therefore, if we only consider the sigma interactions, ML6 complexes should have a molecular orbital diagram like that
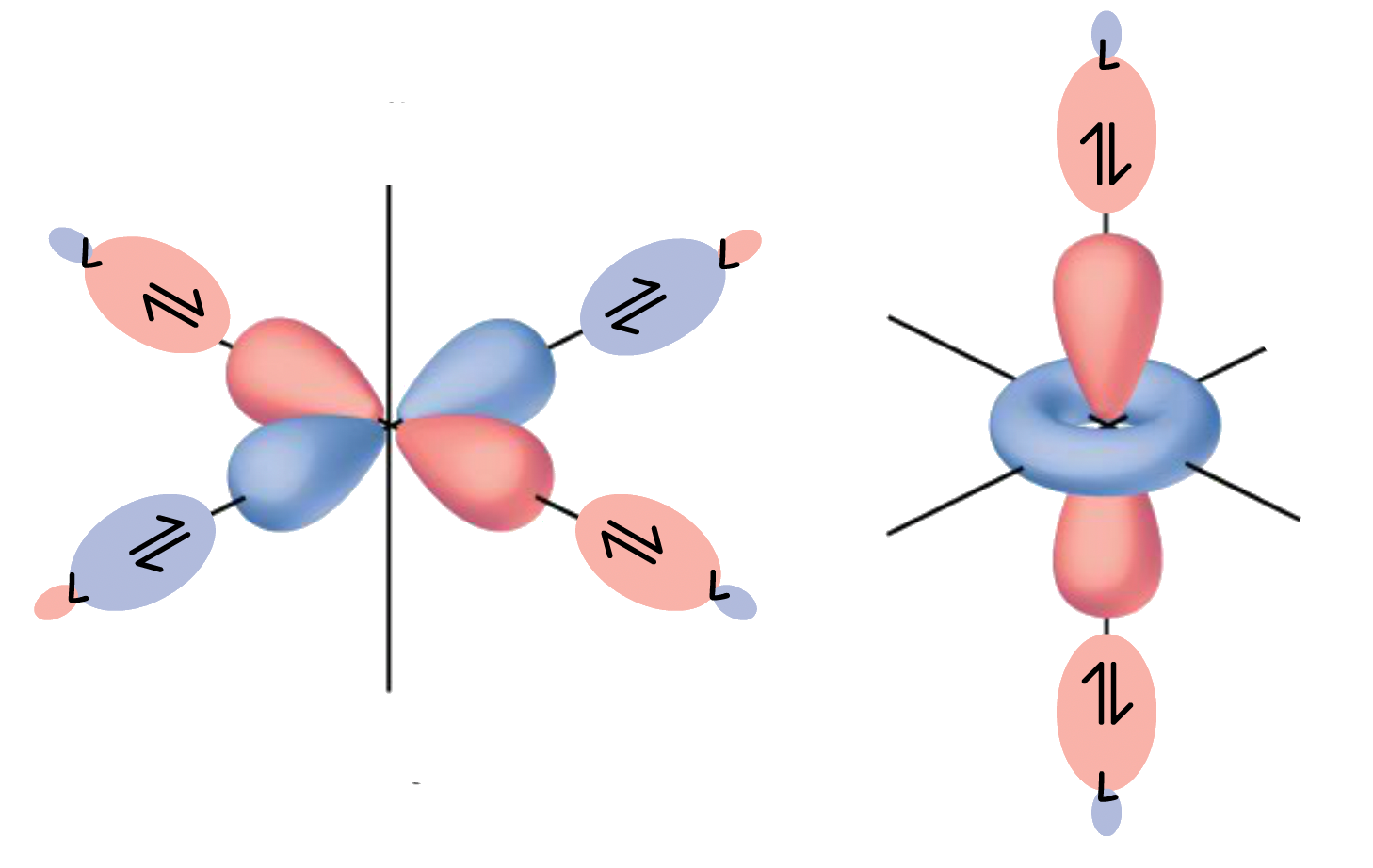
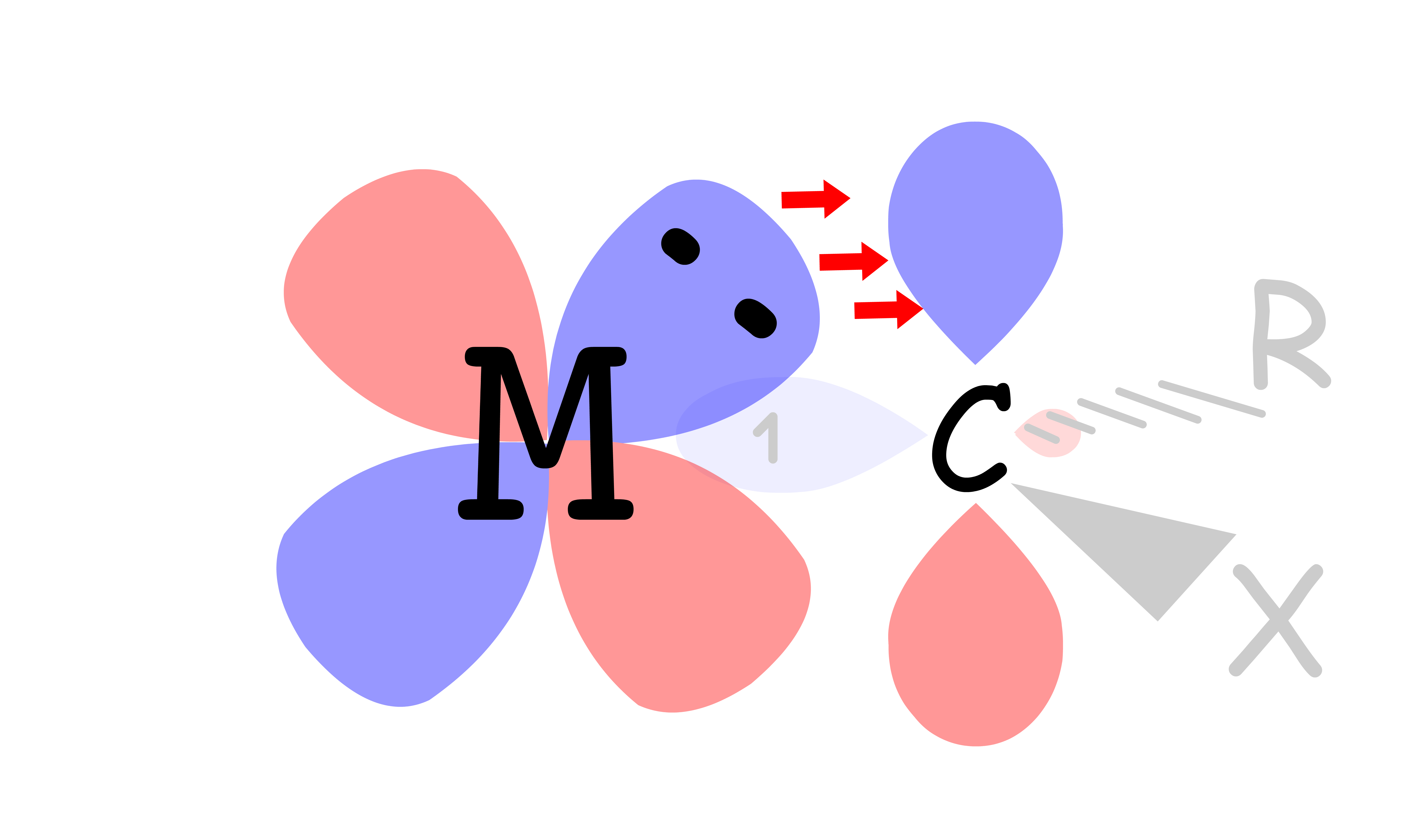
¶ π-Interactions in ML6 Complexes
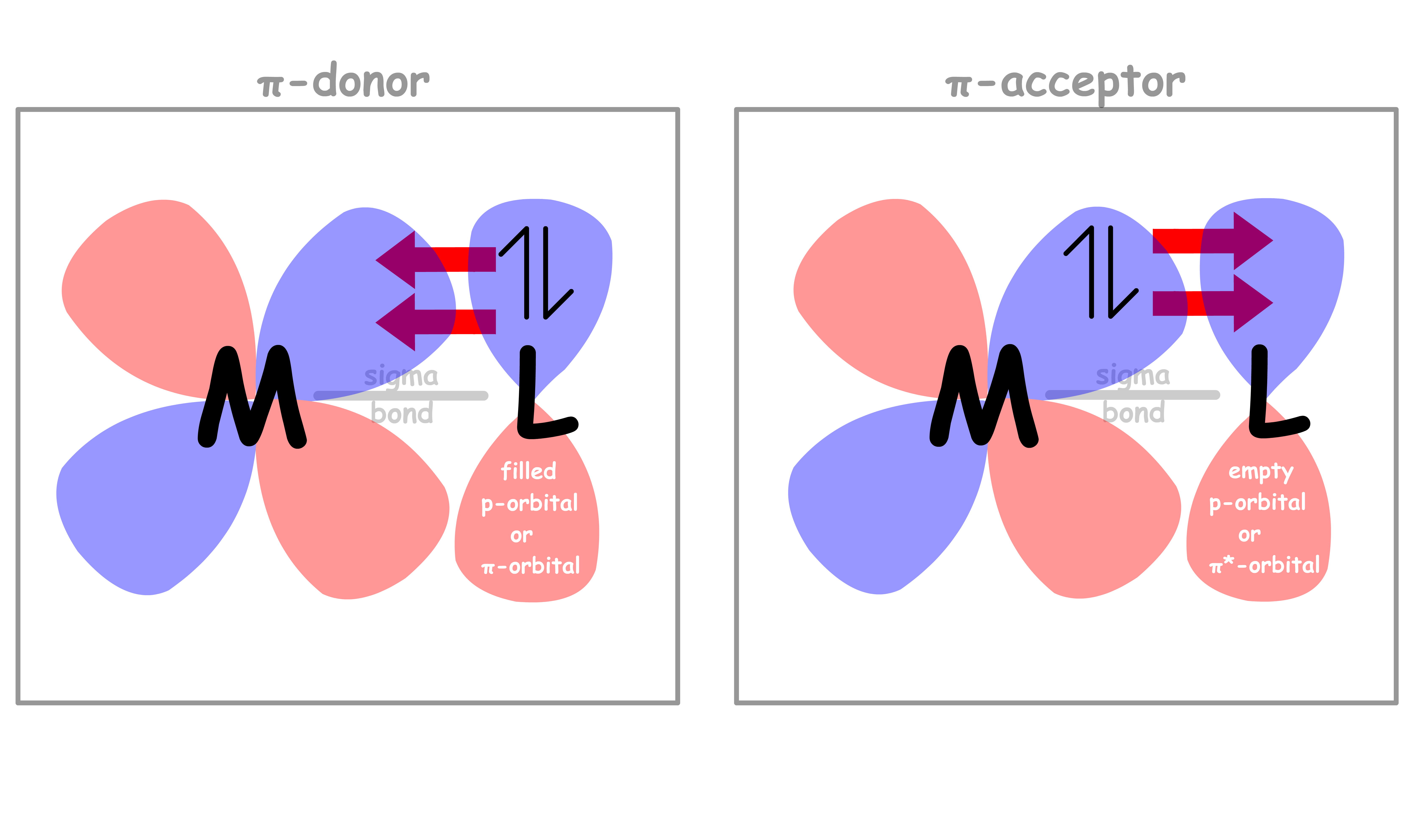
In addition to sigma bonding, some ligands are capable of undergoing π bonding as well
- The orbitals participate in π bonding with the ligands while the orbitals do not
There are two types of π-interactions
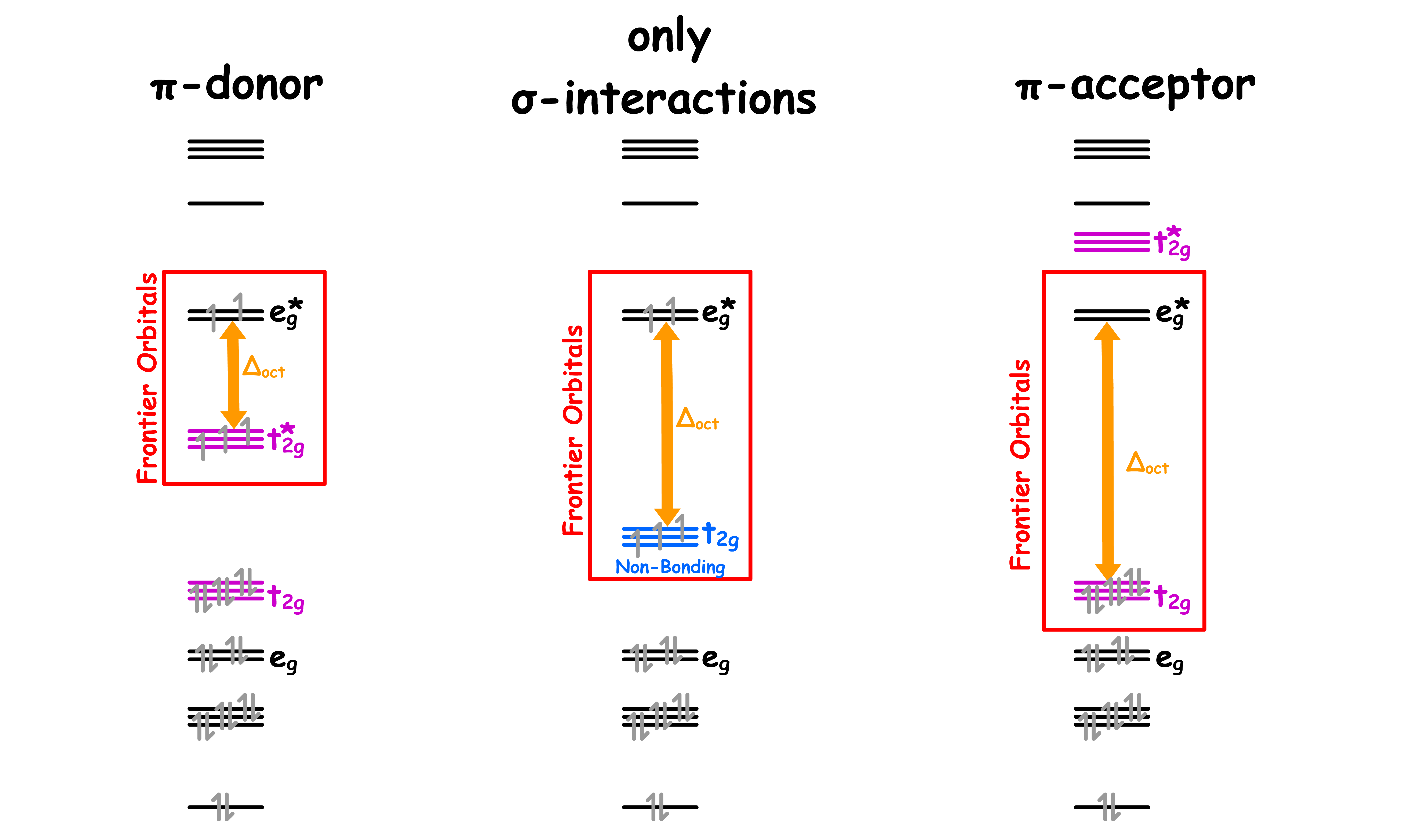
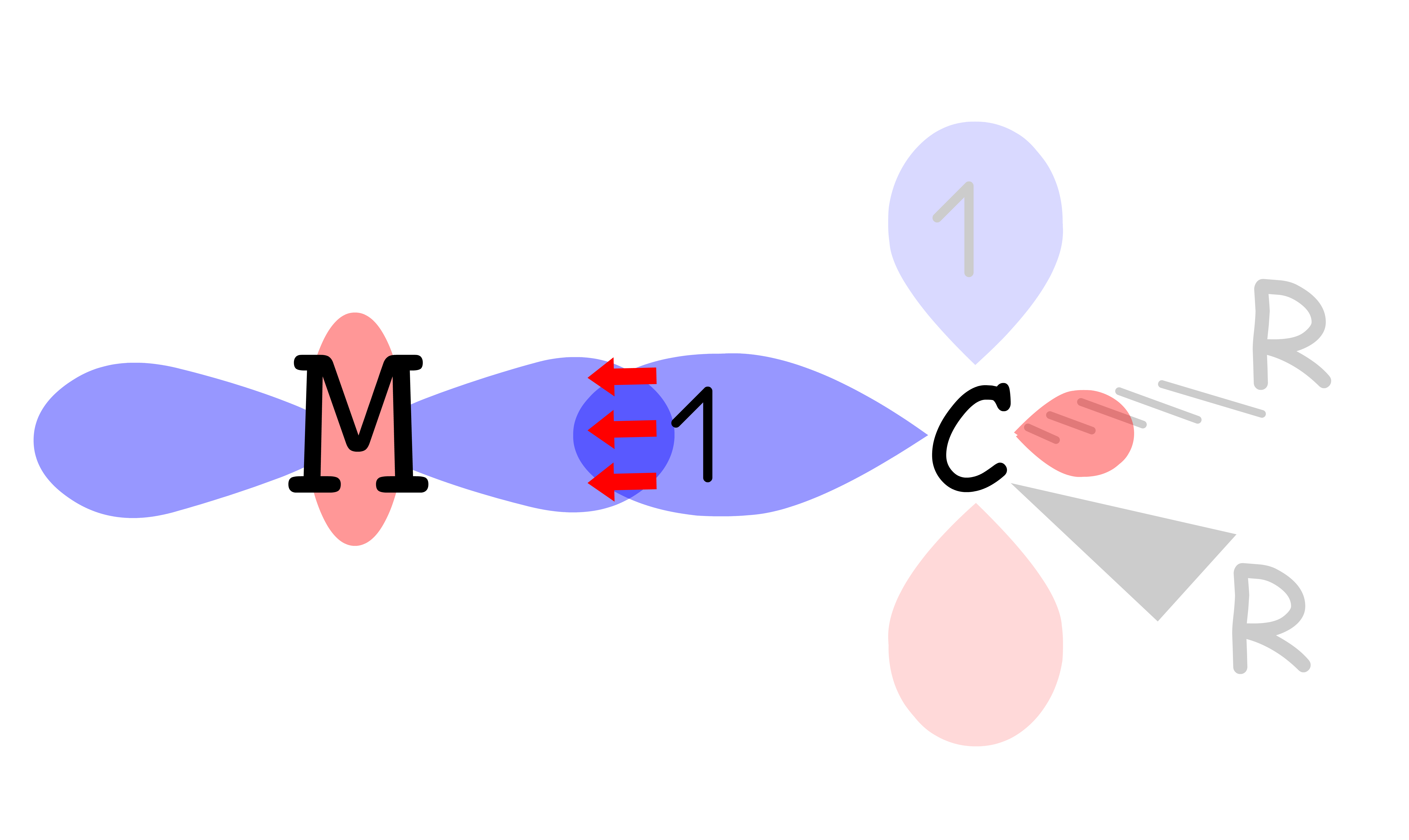
¶ π-donor ligands
- A ligand can be a π-donor if it contains a filled p or π orbtal that is perpendicular to the sigma metal-ligand bond
- The molecular orbital diagram for π-donor ligands can be modified from the MO diagram where only sigma interactions are considered
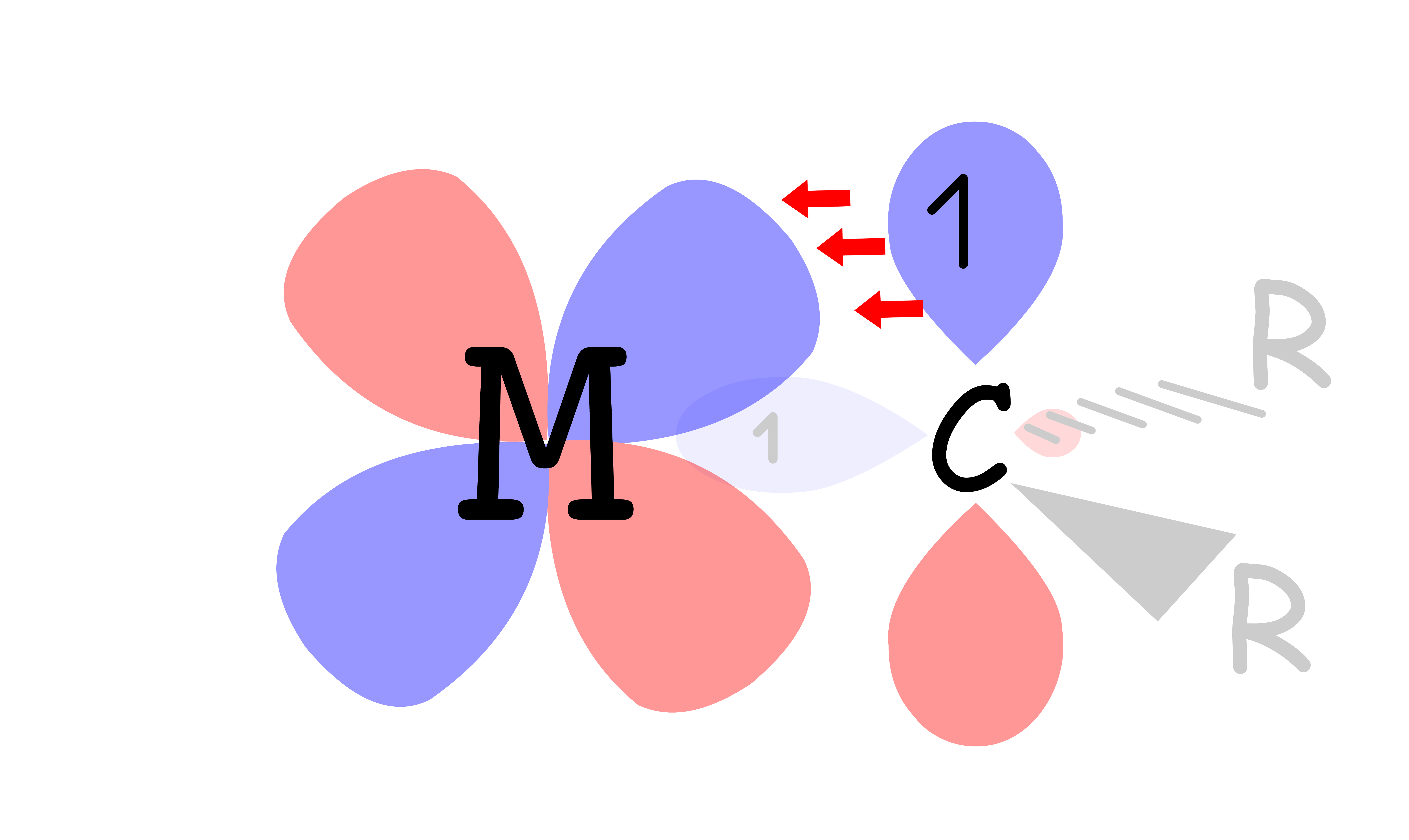
¶ π-acceptor ligands
A ligand can be a π-acceptor if it contains an empty p or π* orbtal that is perpendicular to the sigma metal-ligand bond
The molecular orbital diagram for π-acceptor ligands can be modified from the MO diagram where only sigma interactions are considered
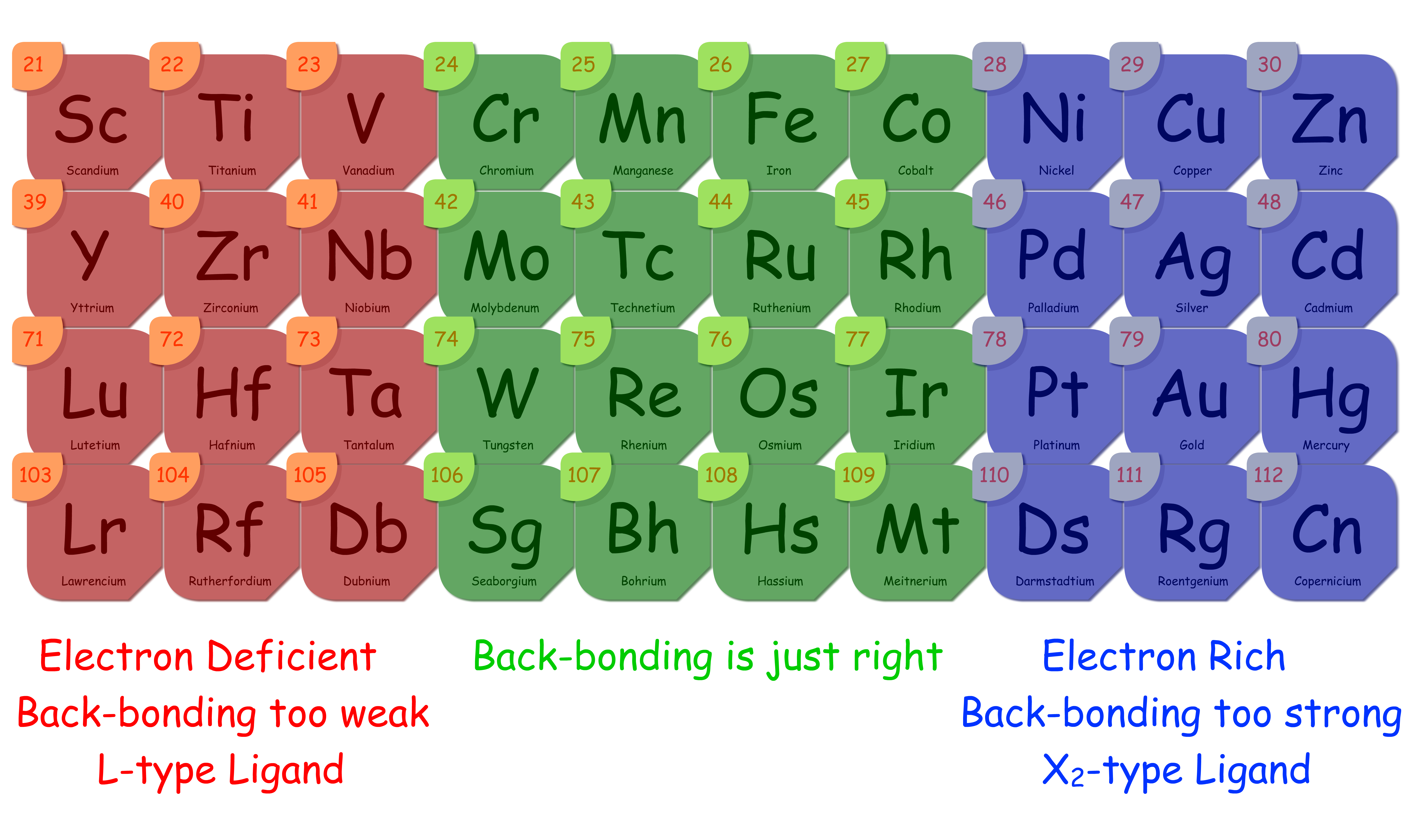
The degree of back donation is related to how electron rich the metal center is
In general, the more electron rich the metal center is, the stronger the π-back donation, the stronger the metal–ligand bond
There are five factors that affects how electron rich a metal centre is
- Oxidation state
- Higher oxidation state = more positive = less electron rich
- Charge of the complex
- If the complex has an overall negative charge, then the metal centre should also be electron rich
- If the complex has an overall positive charge, then the metal centre should be electron deficient
- dn count
- If the total number of electrons is high, then the metal centre is electron rich
- Orbital Overlap Consideration
- Larger orbitals can overlap with the empty π* or empty p orbital better, resulting in a better back donation
- In general, the size of the d orbitals increase down the group (5d>4d>3d)
- Electron-donating or withdrawing effects by other ligands
- If the complex contains electron-donating ligands ( or donors), then the metal centre will become more electron rich
- If the complex contains other π-acceptor ligands, then they will compete with each other for π-back donation, thus weakening the effect
¶ Spectrochemical Series
- Ligands that produce a large splitting are called strong field ligands, and those that produce a small splitting are called weak field ligands
- We can rationalize this trend by examining the molecular orbital diagrams
- We still have exceptions: H- & Me- are high in the series simply because they are strong σ donors ( is very high in energy, so is large)
¶ Number of Electrons
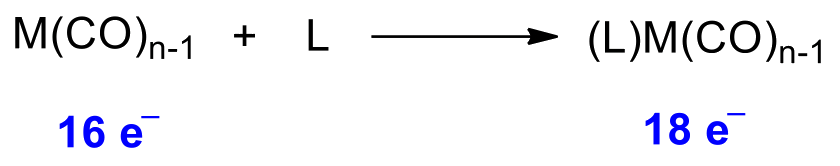
¶ 18 Electron Rule
Stable transition metal complexes tend to have 18 valence electrons around its metal
- This is helpful to predict reactivity and rationalise bonding.
- Of course, we will be looking at some exceptions. In fact, the 18-electron rule is a lot easier to violate than the octet rule
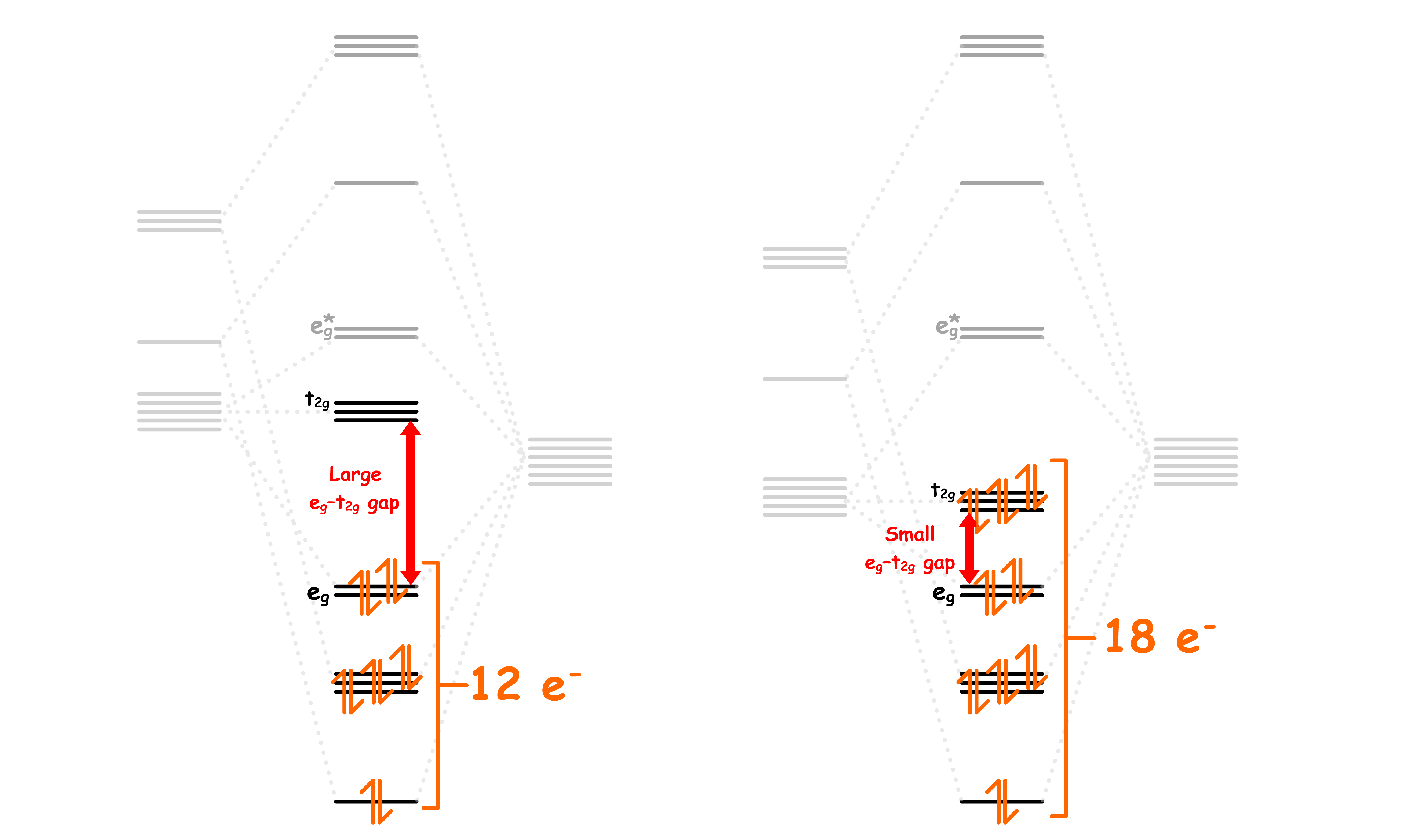
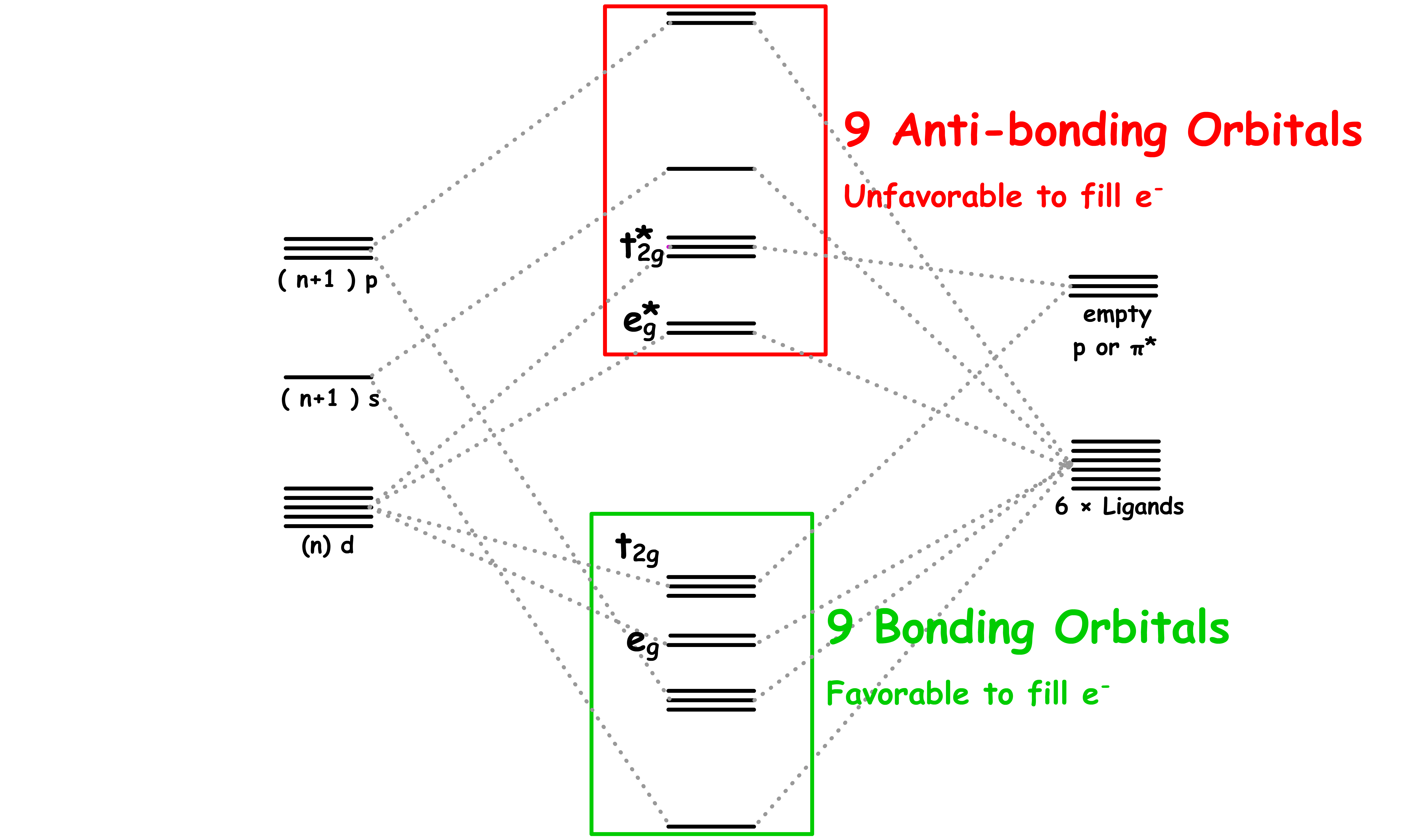
The 18-electron rule applies loosely to octahedral complexes with only sigma interaction
- Consider the corresponding molecular orbital diagram
- Since the bonding orbitals are low in energy, we will always fill up all 6 of them. In other words, there must be at least 12 electrons filled
- The t2g orbitals becomes less favourable to fill as eg–t2g gap increases
We can see that for octahedral complexes with only sigma interactions, the total electron count is either 12 or 18
- Strong σ donor ligands have a large separation, which means it will also have a large separation, making it favorable to fill 12 electrons only
- In any case, prohibits more than 18 e- to be filled as it will decrease stability
The 18-electron rule applies strictly to octahedral complexes with only π-acceptor ligands
- Consider the corresponding molecular orbital diagram
- When the ligands are π-acceptors,
- In the previous case, it is neither favorable nor unfavorable to fill those three orbitals, so filling all 18 electrons is meh
- But now, since the t2g orbital are lowered in energy, there is a reason to fill them
- Large enables t2g to be filled fully and results in kinetic & thermodynamic stability - obeying 18 e- rule
- As a result, the 18 electron rule applies strongly here
Of course, what's the point of setting up a rule if you don't break it. So here are the exceptions:
1. Early transition metal + weak field ligands:
- Fewer electrons in metal atom means sterically impossible to bring electron count to 18
- e.g. [TiF6]2- with 12 e-
2. Late transition metal + weak field ligands:
- Samll , electrons may populate eg* orbital
- e.g. [Ni(NH3)6]2+ with 20 e-
3. Square planar complexes:
- Composed of 4 ligands and metal centre, each contributing to 8 electrons
- Population of all bonding MOs yeilds 16 e- with large
- Note: similar arguments can be used to justify 18 electrons in Td or trigonal bipyramidal
¶ MLXZ formalism
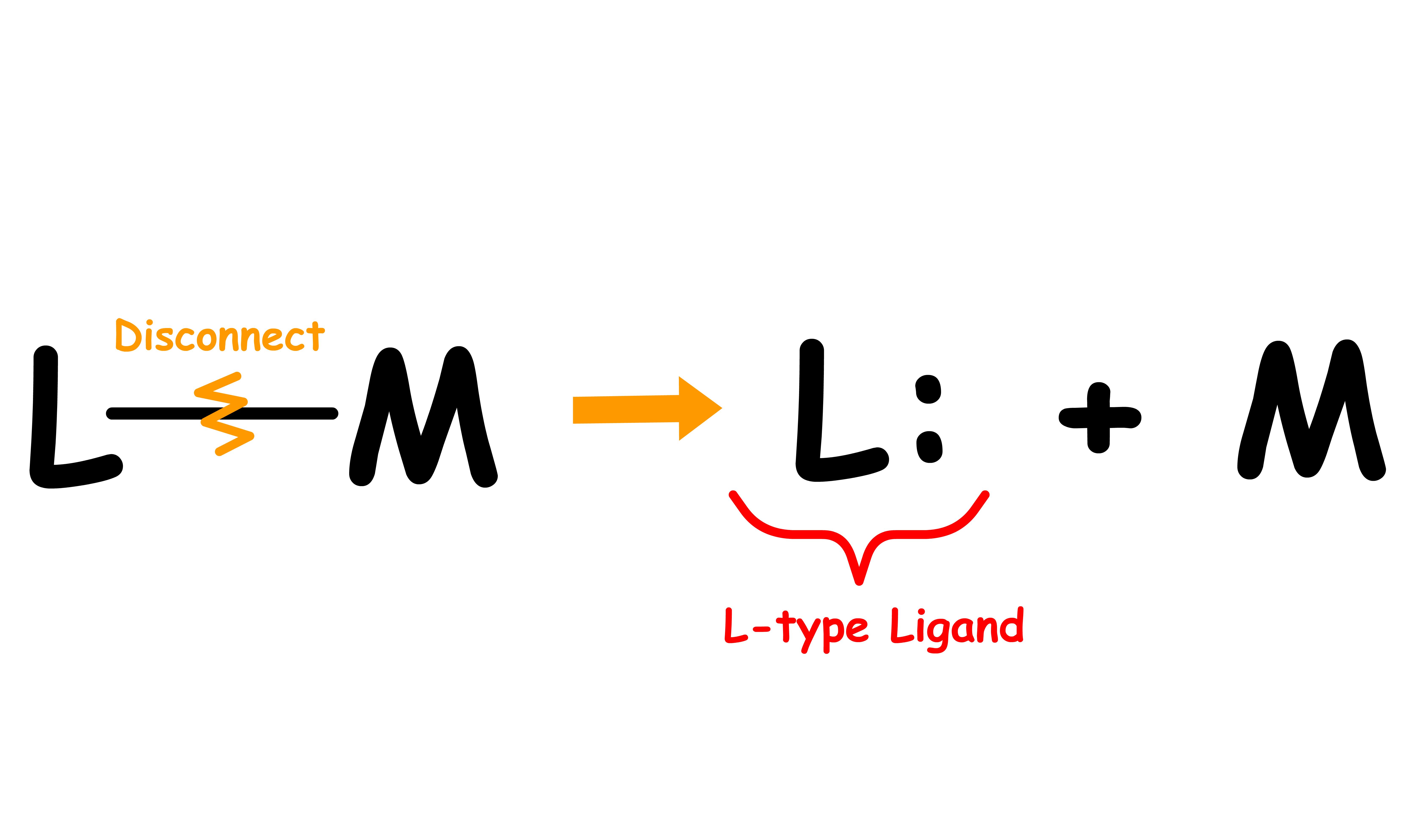
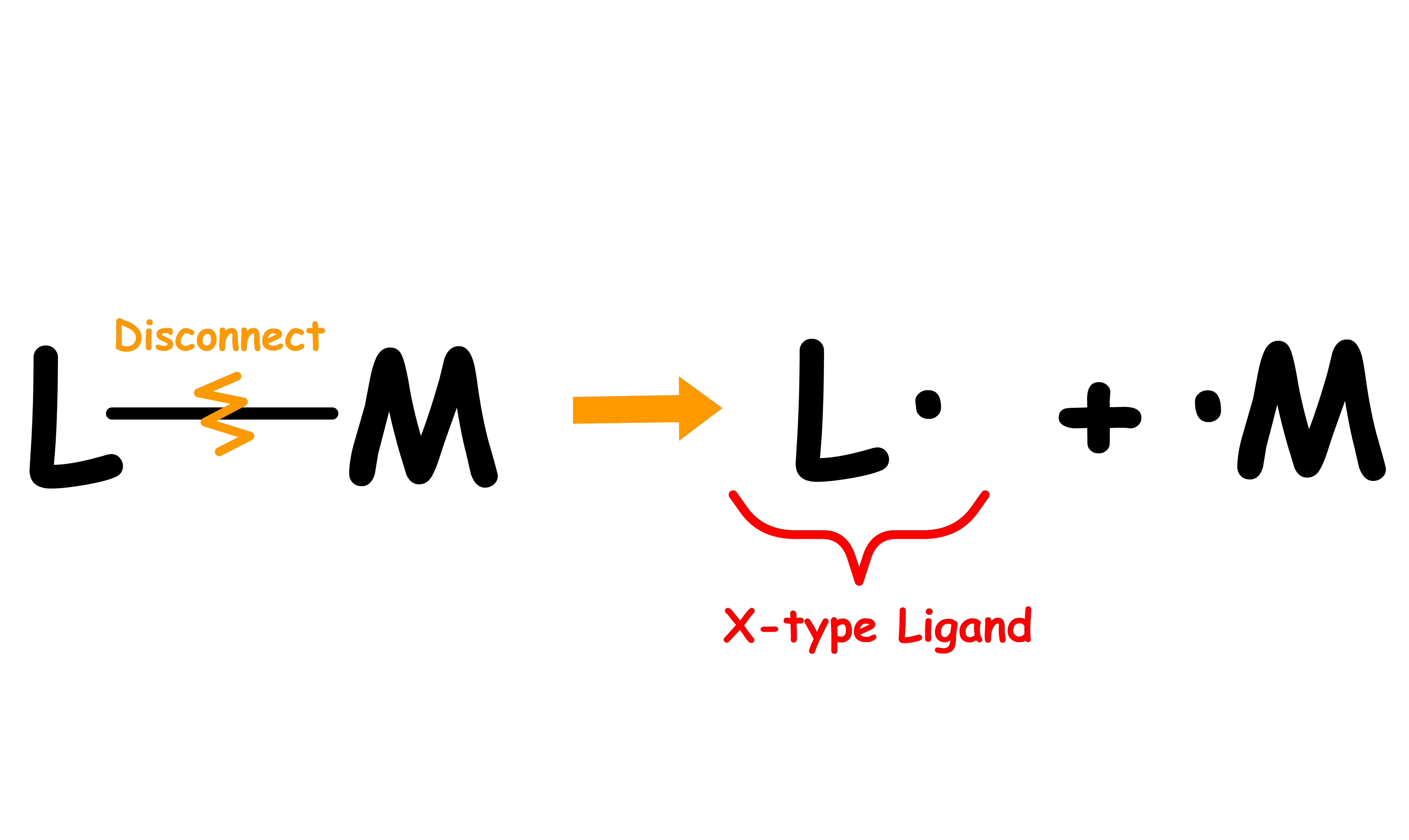
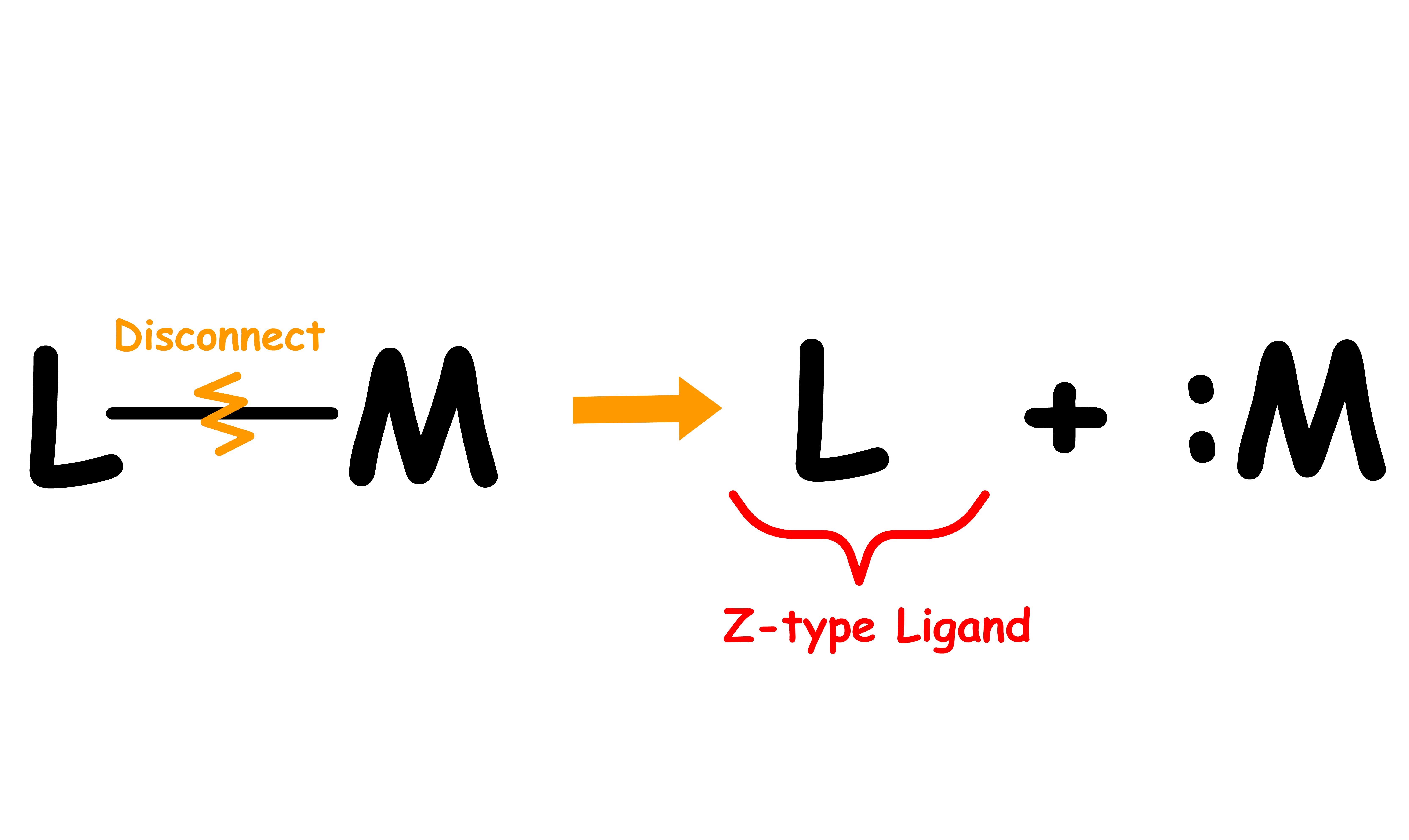
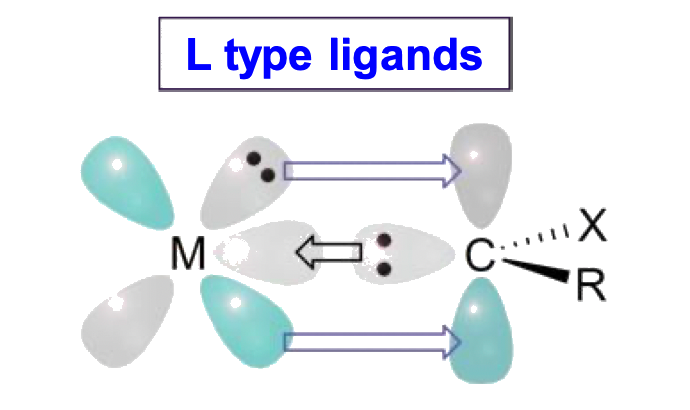
Ligands can be classified into three types: L, X and Z
- We can determine the ligand type by looking at the free state of the ligand
- Disconnect the metal-ligand bond and reassign the bonding electrons in a way that allows the disconnected ligand to be neutral
- The ligand type depends on how many electrons are assigned to the disconnected ligand
- If both bonding electrons are assigned to the disconnected ligand to make it neutral, the ligand is an L type
- If only one bonding electrons are assigned to the disconnected ligand to make it neutral, the ligand is an X type
- If none of the bonding electrons are assigned to the disconnected ligand to make it neutral, the ligand is an Z type
We can also determine the ligand type by considering the number of electrons the ligand is donating to the metal center
- L-type ligands are 2-electron donors
- X-type ligands are 1-electron donors
- Z-type ligands are 0-electron donors
This method allows us to determine the ligand type for ligands that contribute more than two electrons
It is easier if I just give a few examples:
- E.g. If the ligand is donating 6 electrons, it may be a ligand
- E.g. If the ligand is donating 5 electrons, it may be a ligand
¶ Bridging complexes:
There are four main ways to bridge two complexes, each contributing to different electron count
L type:
- A total of 2 electrons
- to each metal centre
X type:
- A total of 3 electrons
- and to each metal centre
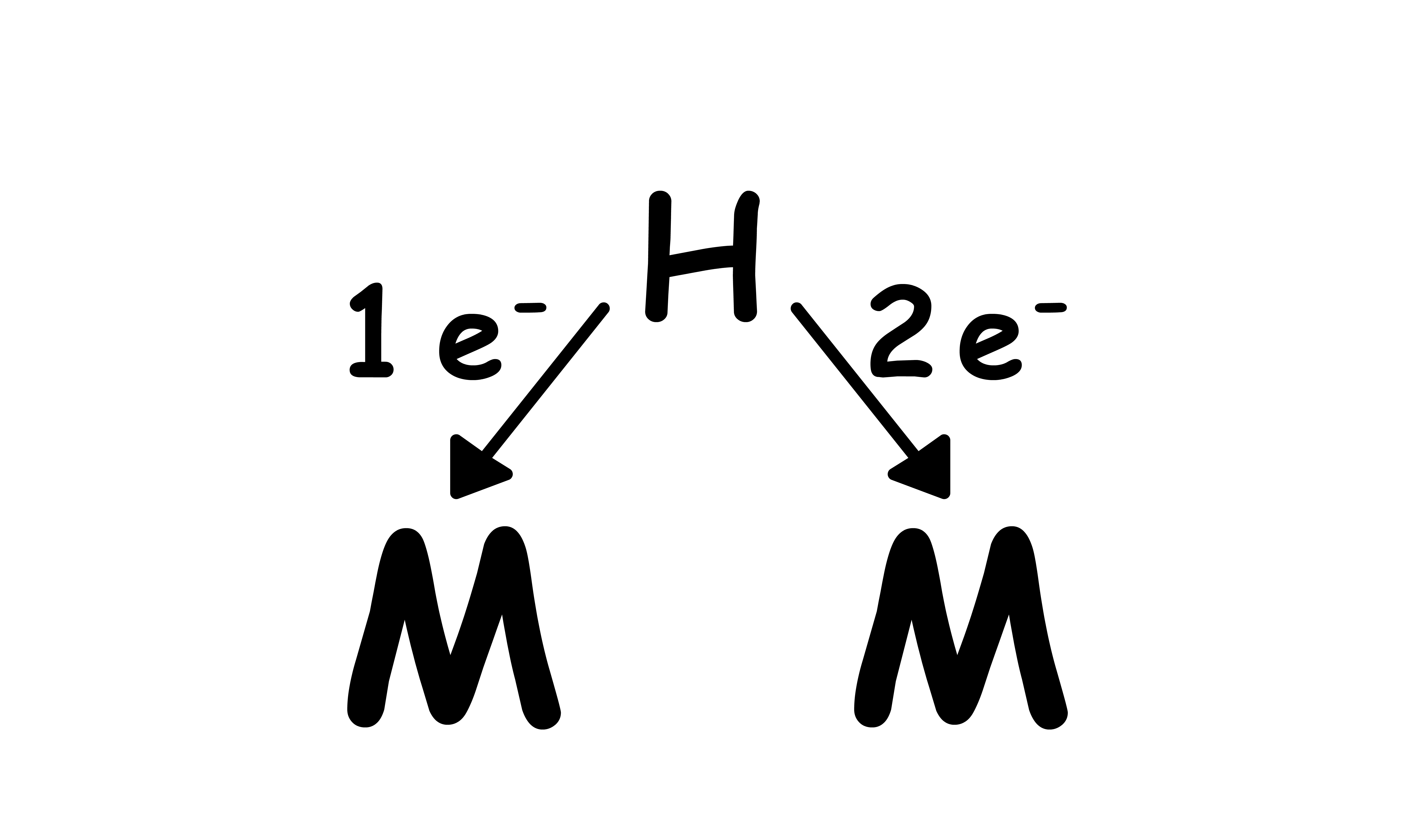
Hydride:
- A total of 3 electrons
- and to each metal centre
- In agostic bond
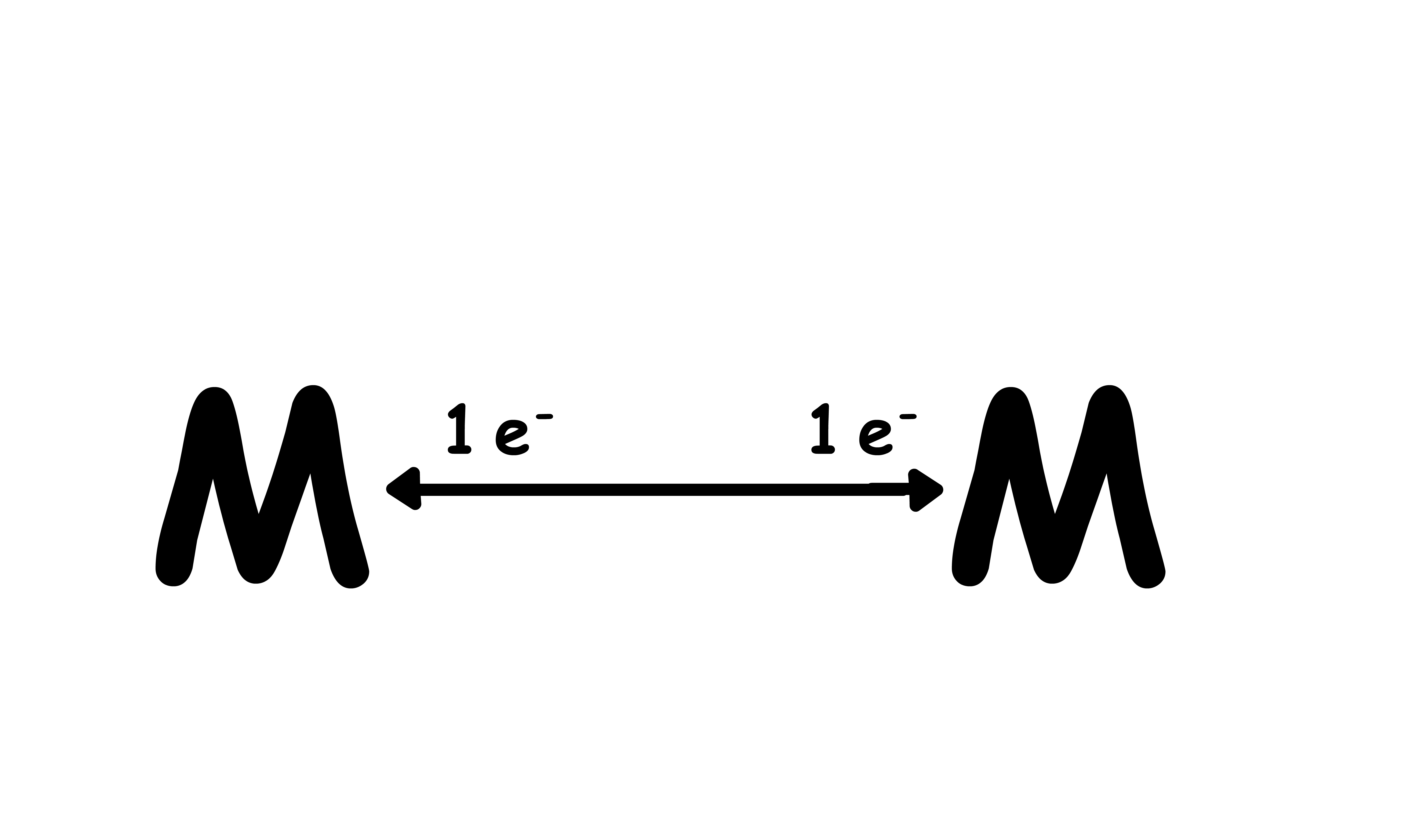
Metal-metal bond:
- A total of 2 electrons
- to each metal centre
Terminologies
- A detailed explanation is in the year 1 notes: Transition-Metals
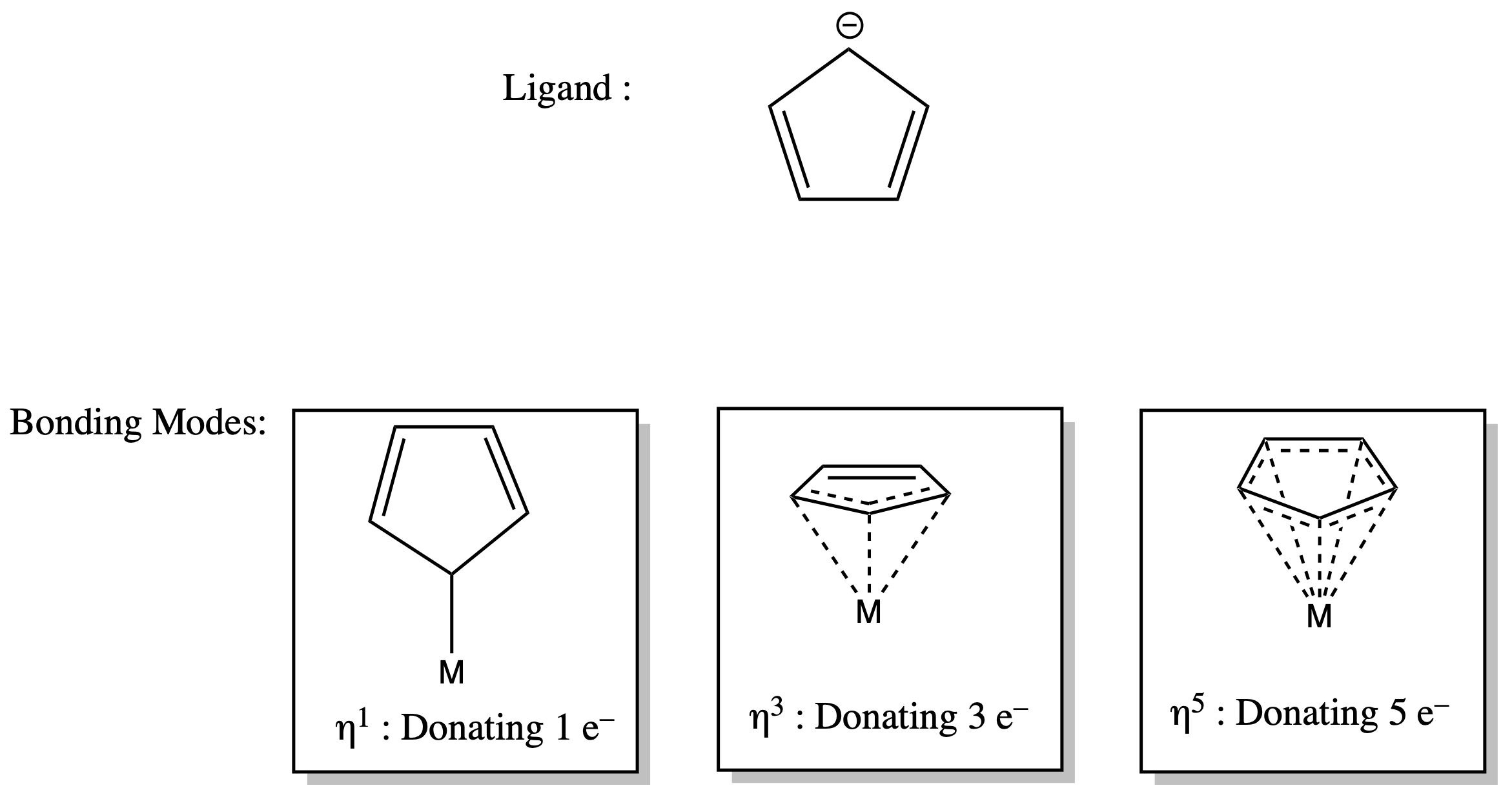
ηn ( hapticity )
- the number of atoms of the ligand (n) bound to the metal centre
μn
- denotes a ligand bound to (n) number of metal atoms
¶ Electron Counting and Determining Oxidation States
We can count the total number of electrons by following these steps
Step 1 - Count the number of electrons contributed by the Metal
- We consider the metal to always be neutral, so that the number of electrons contributed by the metal is just the group number of said metal
- The oxidation state of the metal does not matter for this calculation!
Step 2 - Count the number of electrons contributed by the Ligands
- L-type ligands contribute 2-electrons
- X-type ligands contribute 1-electrons
- Z-type ligands contribute 0-electrons
Step 3 - Account for the charge of the complex
- Add one electron for each negative charge
- Remove one electron for each positive charge
- Do nothing if the complex is neutral
We can determine the oxidation state of the metal by following these steps
Step 1 - Count the number of X-type ligand
Step 2 - Count the charge of the complex
Step 3 - Use this formula
¶ Metal Carbonyl
CO is an extremely common π acceptor ligand in transitional metal complex
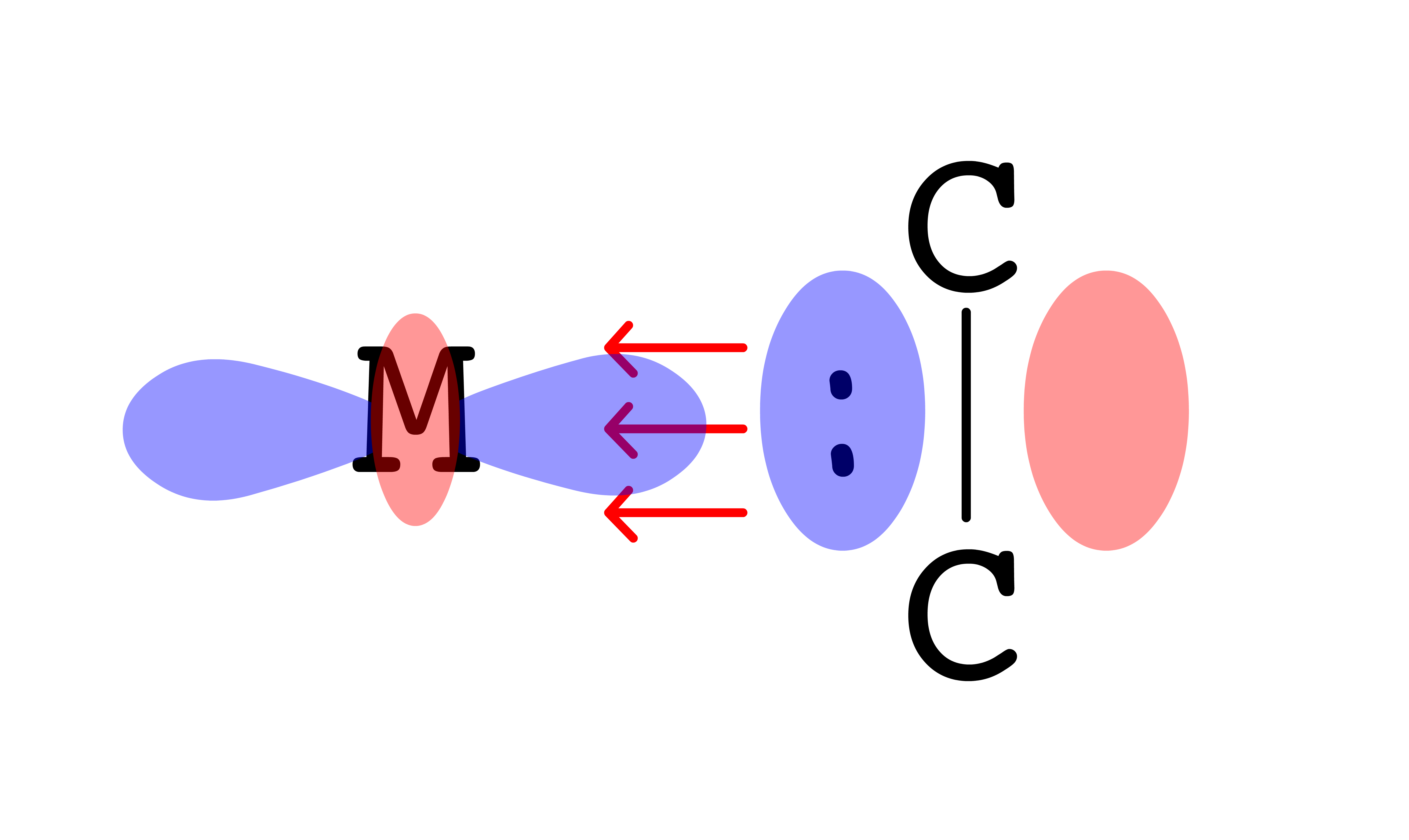
- Both the carbon and oxygen has a lone pair, but carbon is bearing a negative charge. Hence, CO coordinates to the metal centers from the carbon end
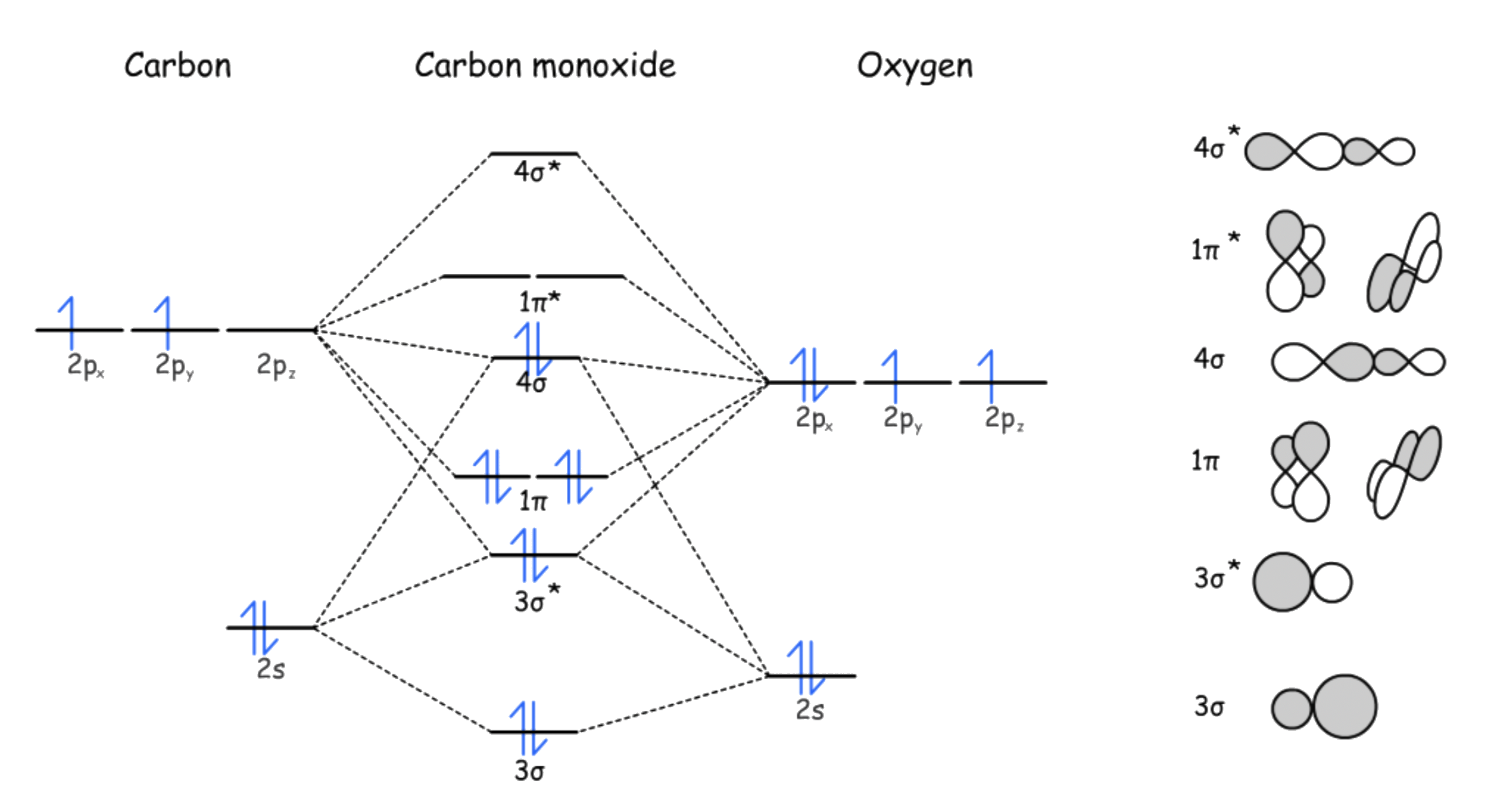
The molecular orbital diagram of CO is given as such
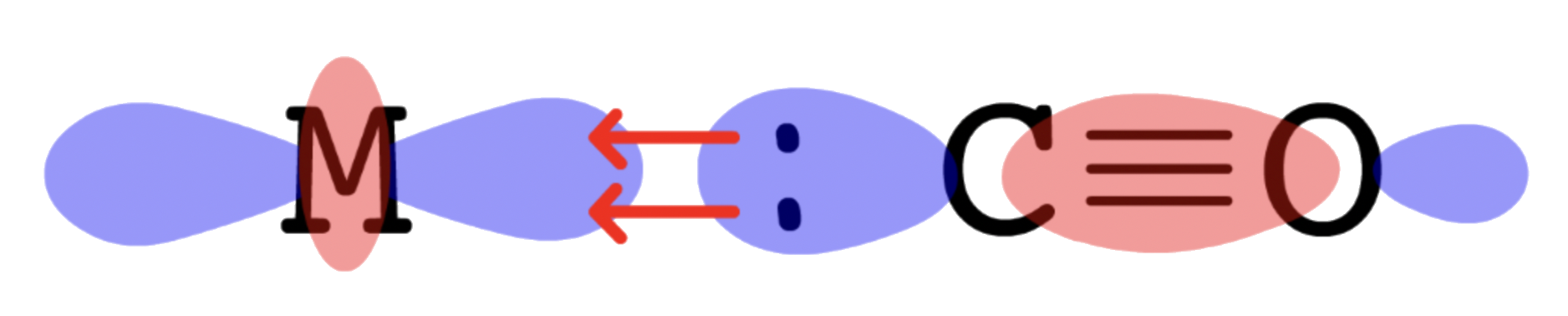
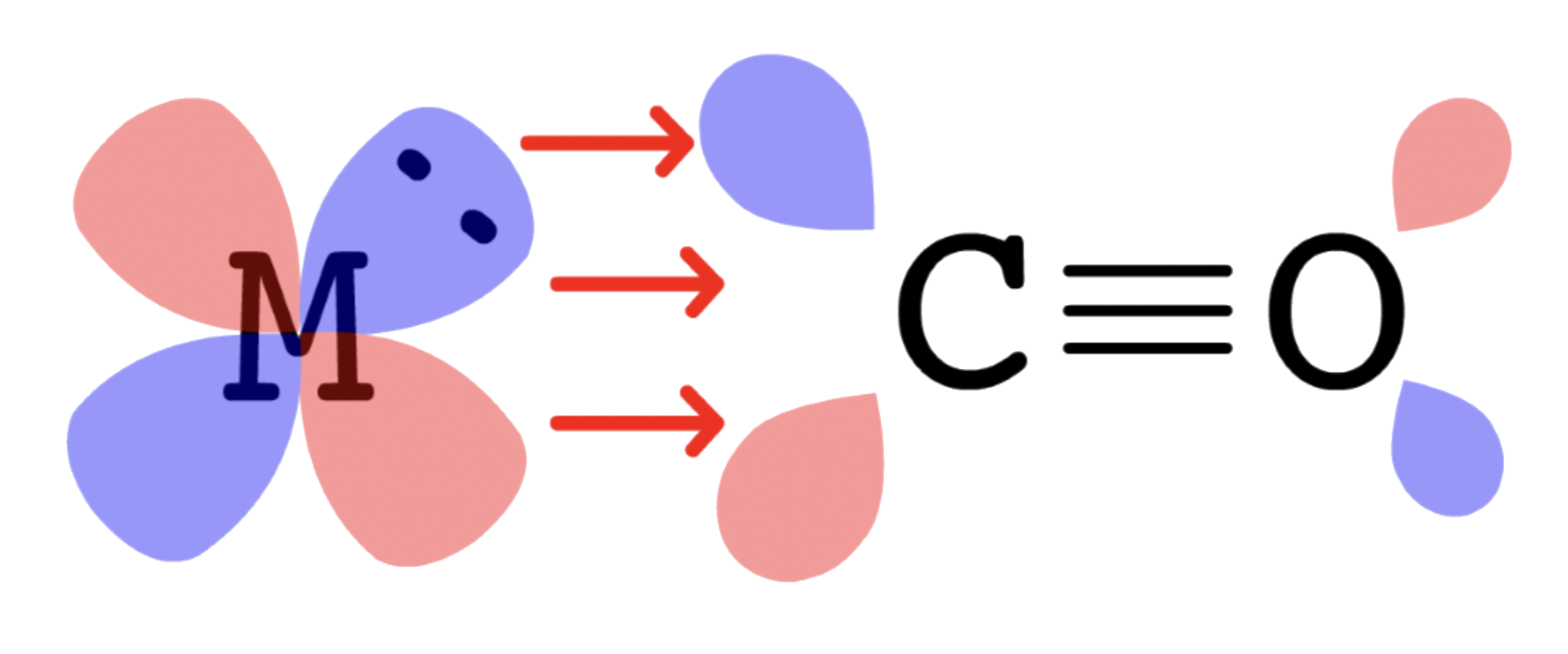
- The HOMO of CO is , so the electron pair will be donated from to a vacant orbital on M ( interactions )
- The LUMO of CO is , so the electron pair will go from the filled orbital of M back to of CO ( interactions )
- Since for the sigma and pi interactions are linear, the CO is bonded to the metal center in a linear fashion
The π-back-bonding weakens the CO bond for two reasons
- The HOMO of the CO is a bonding orbital, the donation of electron pair from the bonding orbital will lower its bond order, thus weakening the CO bond
- The LUMO of the ligand is an antibonding orbital, the acceptance of electrons will lower the bond order, thus weakening the CO bond
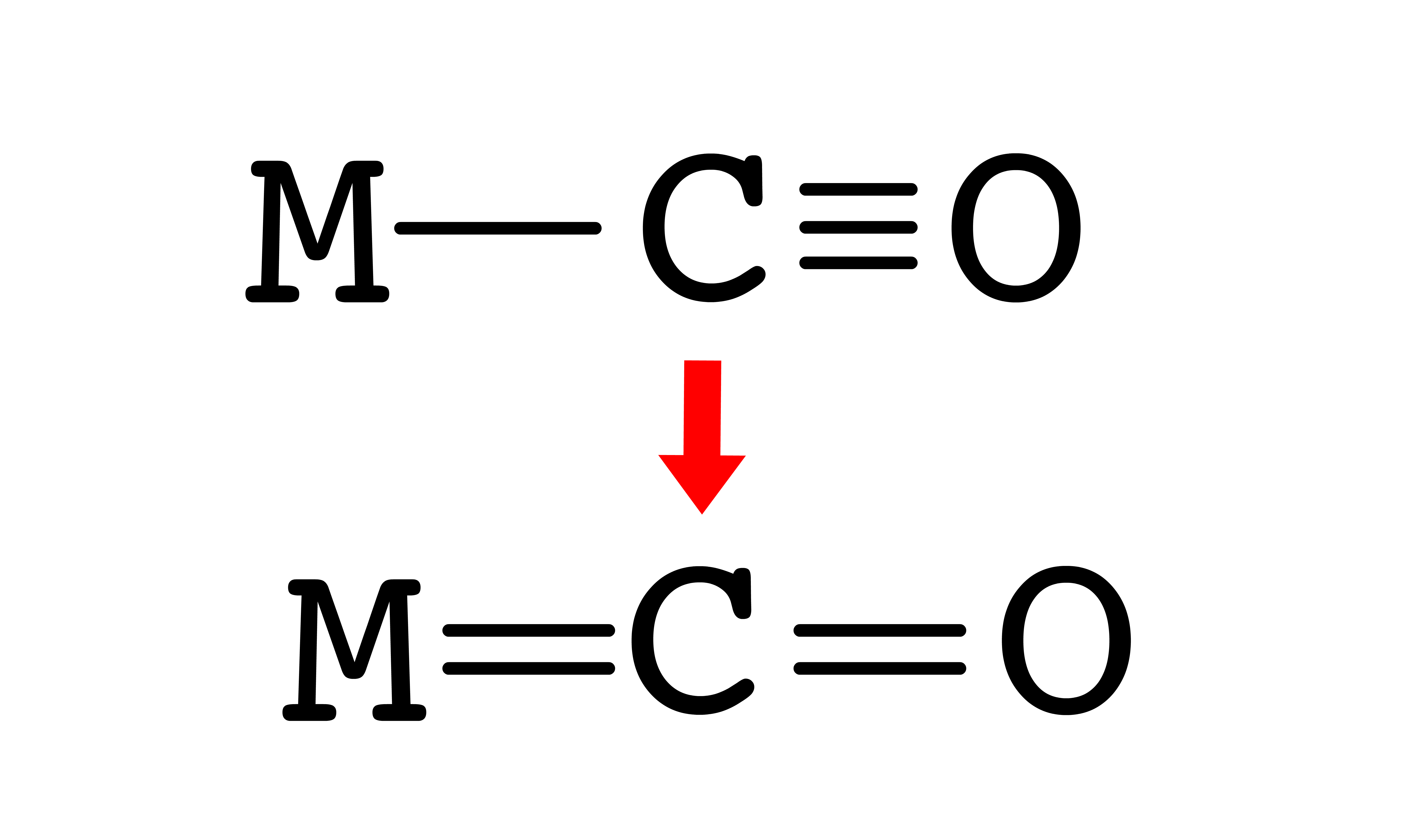
As C–O bonding decreases, M–C bonding increases
- This cause the CO triple bond to revert to CO double bond and causes the MC single bond to become a MC double bond
¶ Synthesis of Metal Carbonyl
¶ Reductive carbonylation
- CO stabilises low oxidation states
- Avoid water as H2O molecules can act as a ligand and bind to the metal center, and reacts with reducing agents subsequently
¶ Metal + CO gas
- Unique to Ni and Fe
- Fe must be specially prepared (free of surface oxides) and high
temperature or high pressure is required
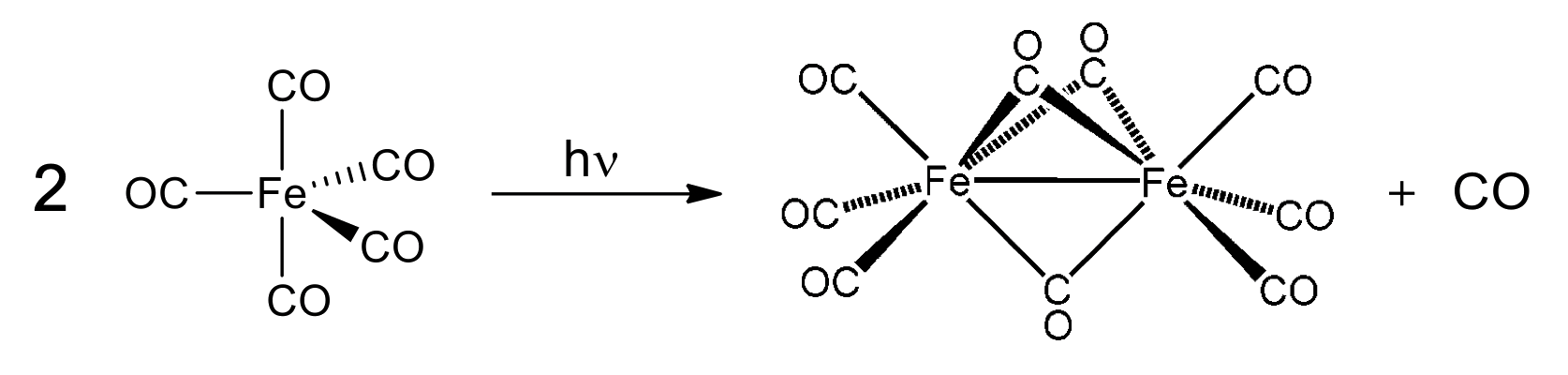
¶ Photochemical/Thermal
- UV light/heat used to remove CO from existing M(CO)x species and generate higher nuclearity (more metal centres) carbonyl species
¶ Reactions of Metal Carbonyls
¶ Substitution
- The reaction is usally carried out in a step-wise fashion
Step 1 – Dissociation / Decarbonylation
Step 2 – Addition / Coordination
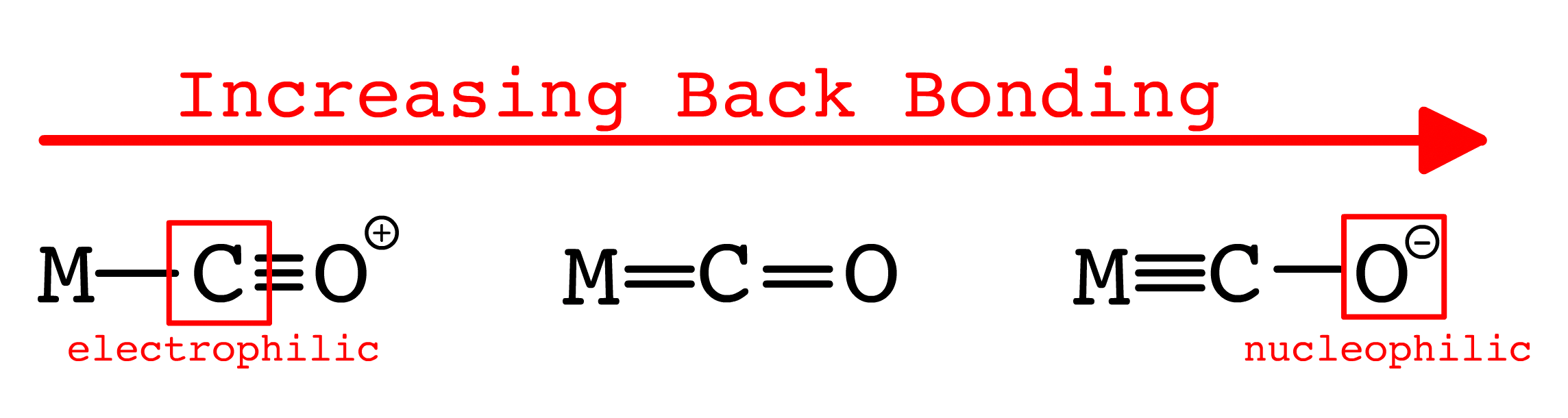
¶ Electrophilic and Nucleophilic attack
- Coordinated CO is more reactive than free CO
- The coordinated CO can either behave as a nucelophile or an electrophile depending on the strength of the π-back bonding
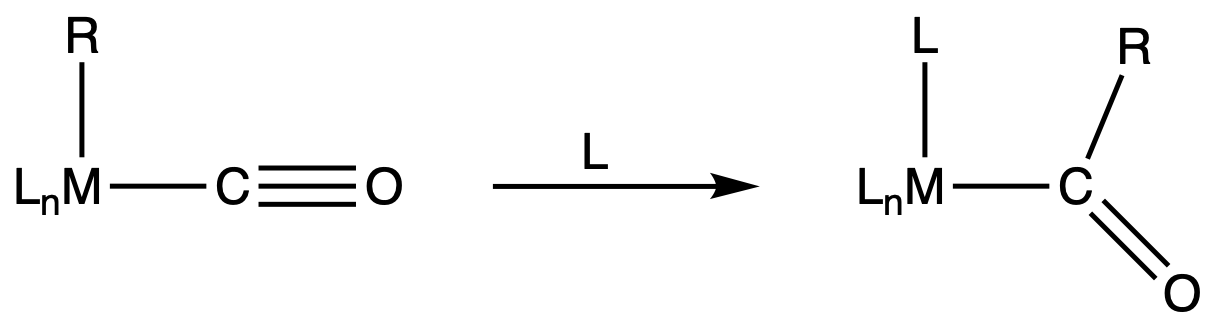
¶ Migratory insertion
- No change in oxidation State
- CO group must be cis (adjacent) to the nucleophilic R group
¶ Metal Complexes of Alkenes and Allyls
Alkenes and other conjugated π systems can act as ligands
- The HOMO of alkene is the orbital, so the electron pair will be donated from the bond to a vacant orbital on M ( interactions )
- The LUMO of alkene is , so the electron pair will go from the filled orbital of M back to of alkene ( interactions )
- The general consensus is that the –bonding is weak and overall,
the bonding is dominated by –back-bonding
The π-back-bonding weakens the C=C bond for two reasons
- The π orbital of the C=C is a bonding orbital, the donation of electron pair from the bonding orbital will lower its bond order, thus weakening the C=C bond
- The LUMO of the C=C is an antibonding orbital, the acceptance of electrons will lower the bond order, thus weakening the C=C bond
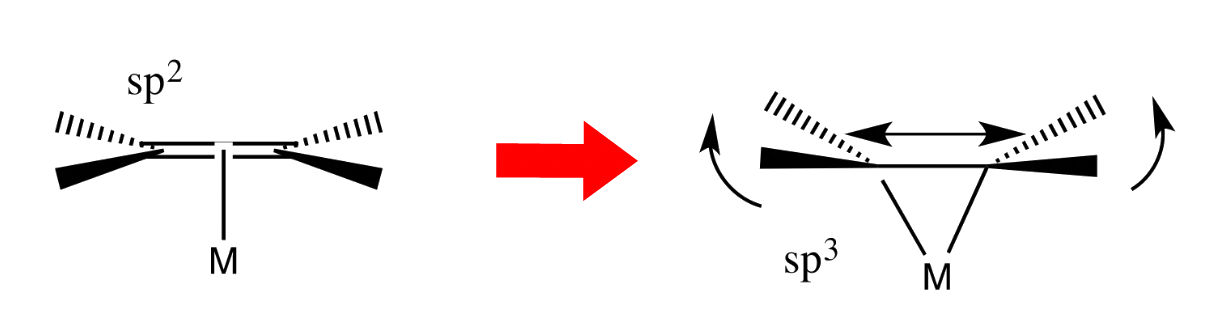
As C=C bonding decreases, M–C bonding increases
- This cause the ligand to behave less like an alkene and more like an alkane
- This is reflected from the fact that the carbons slowly transitions from sp2 to sp3 as the π-back donation increases
¶ Hapticity and Conjugated Systems
Conjugated π systems have the ability to donate more than two electrons to the metal centre simultaneously
- For this reason, they can have different bonding modes (donating different number of electrons)
- The bonding mode these ligands adopt usually depends on the number of electrons the complex needs
¶ Metal Carbenes
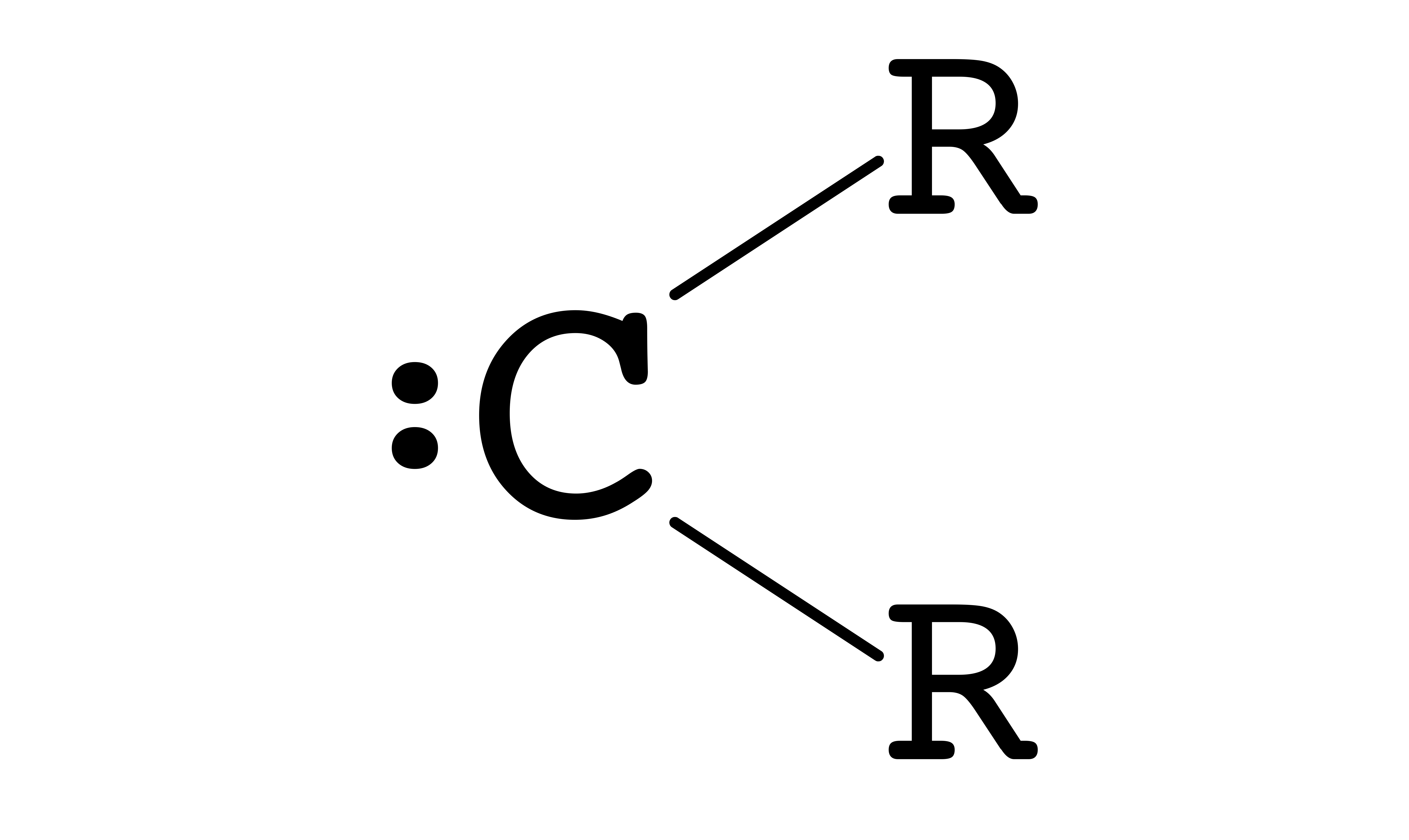
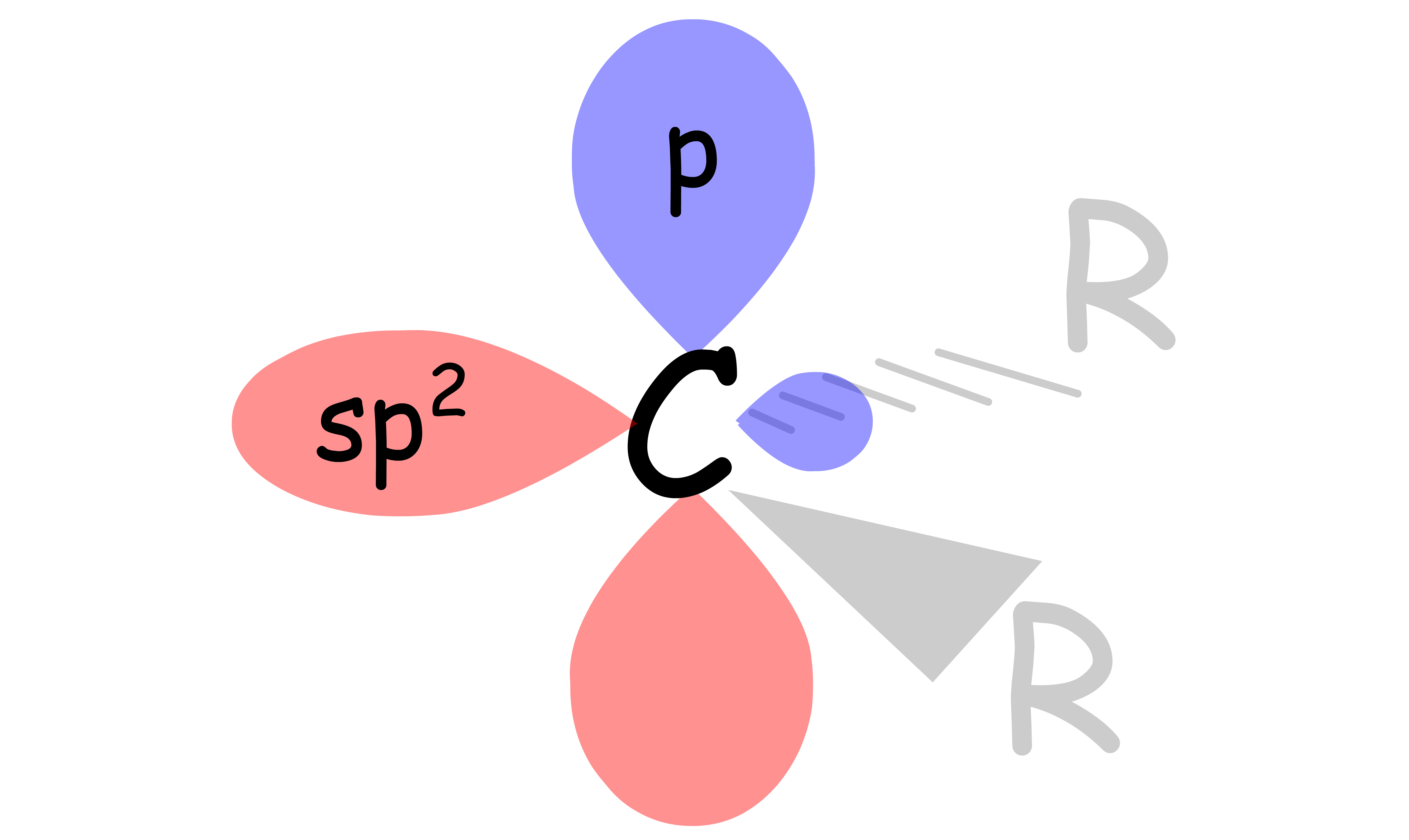
A carbene is a molecule containing a neutral carbon atom with two unshared valence electrons
- Carbenes only have 6 valence electrons, so it does not obey the octet rule, making it unstable
- The geometry of carbene is trigonal planar, so the hybridization of the carbene carbon is sp2
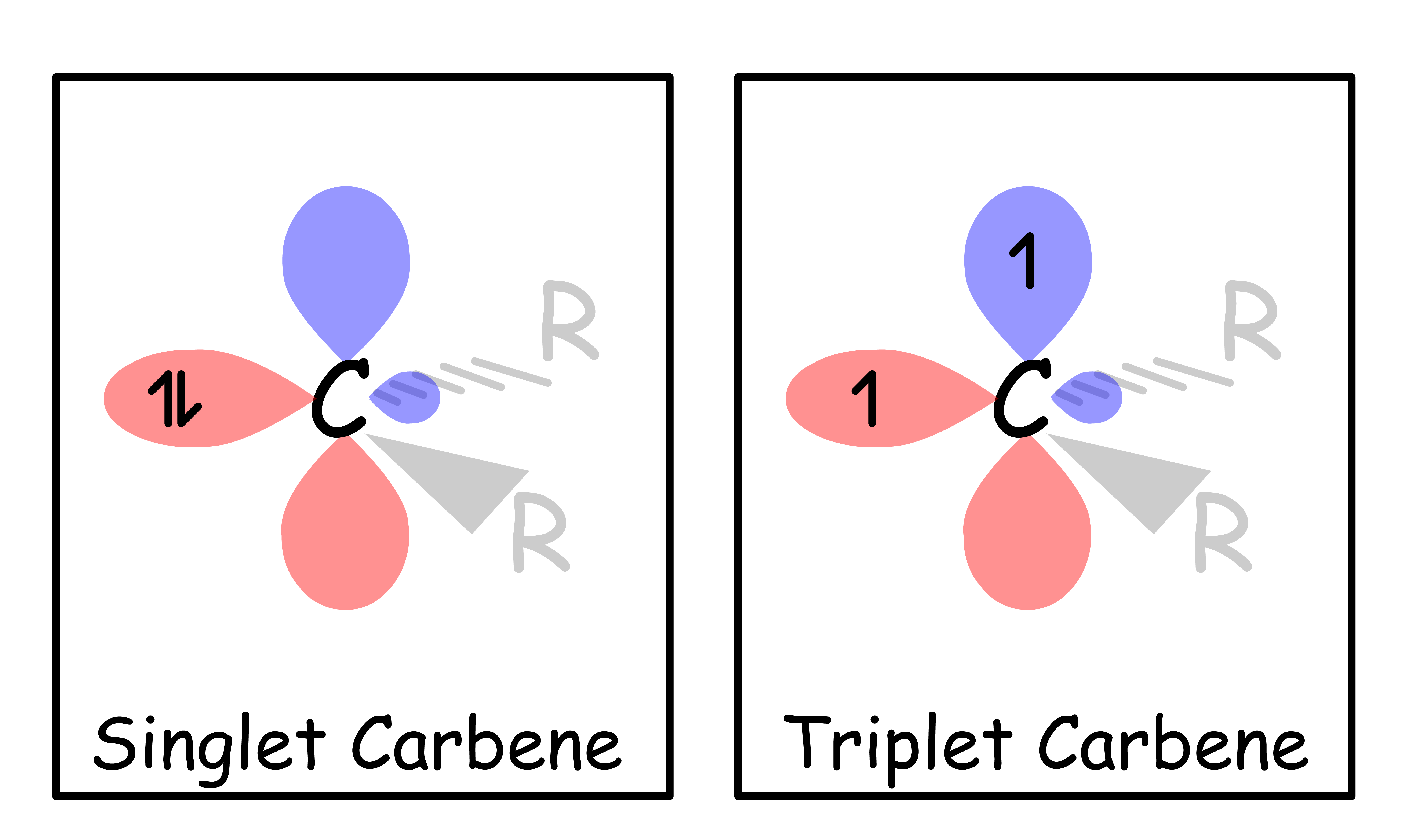
- There are two types of carbenes, they differ in their way of filling electrons
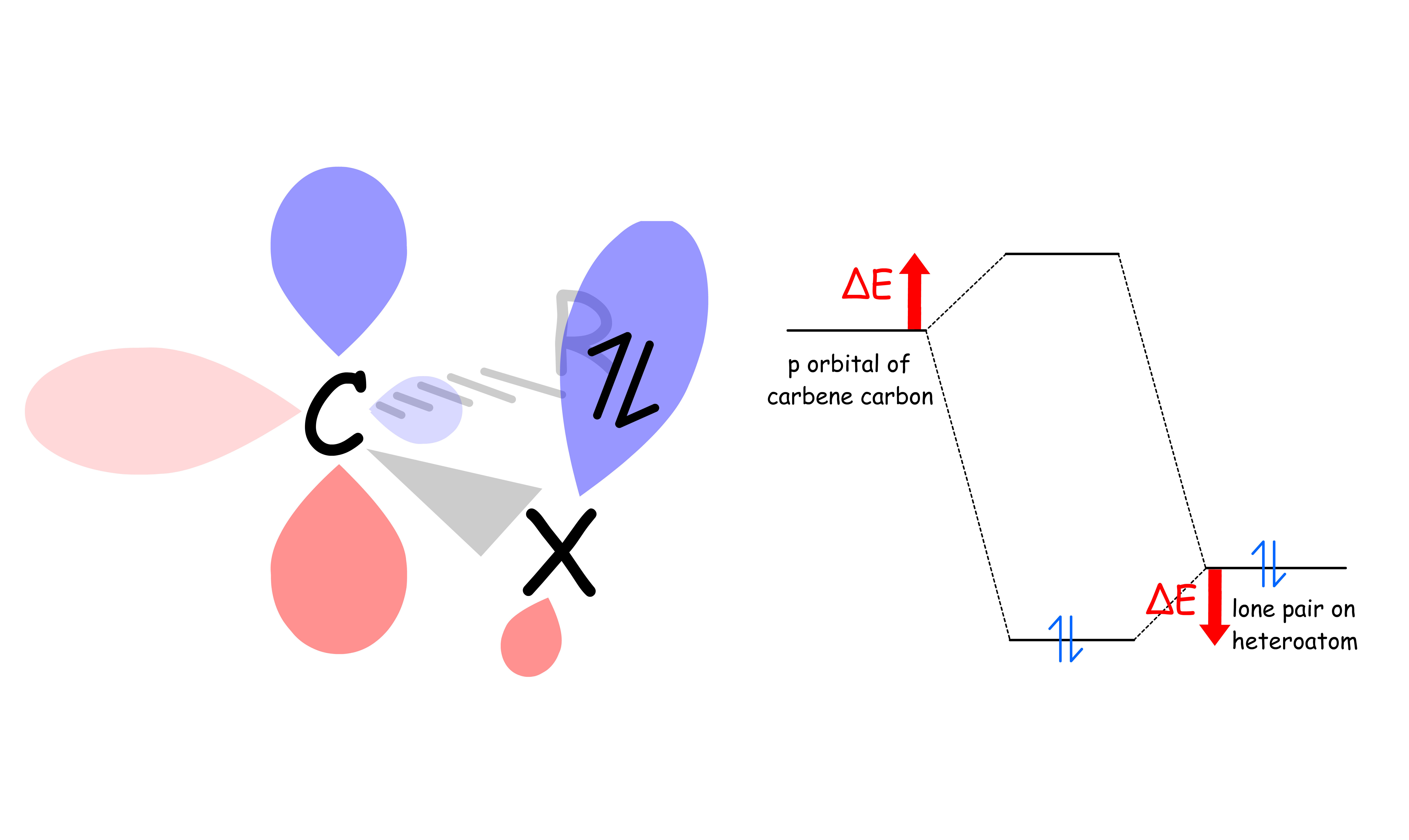
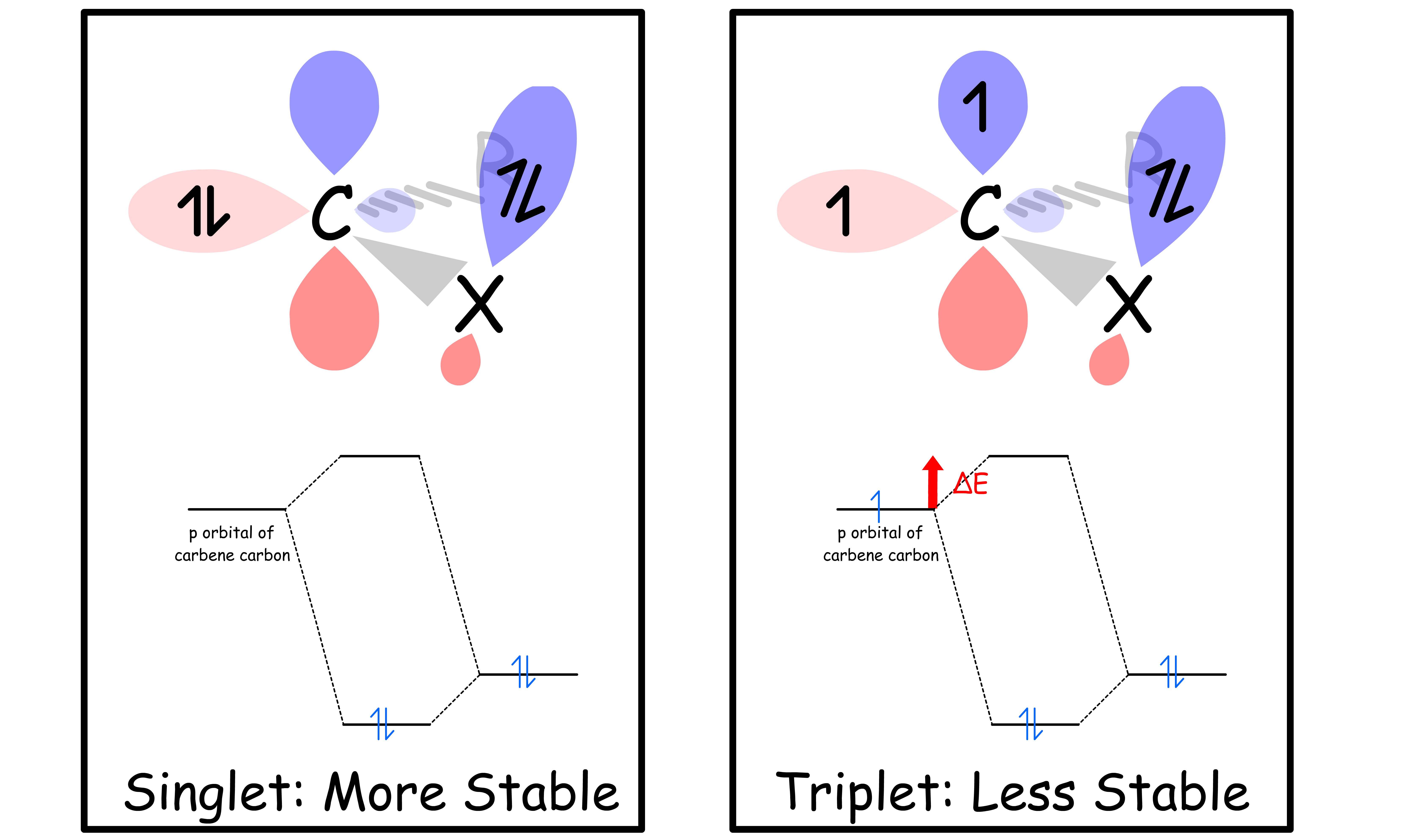
Which electron configuration a carbene adopts depend on whether there's a heteroatom adjacent to the carbene carbon
- This conjuation stabilizes singlet carbene more than triplet carbene
- In both cases, the lone pair on the heteroatom is stabilized
- In triplet carbene, one electron is inhabiting the p orbital, so it will be destabilized upon conjugation
- In singlet carbene, no electron is inhabiting the p orbital, so it will not suffer from that issue
We can therefore conclude
- If there is a heteroatom adjacent to the carbene carbon, the carbene is a singlet
- If there is no heteroatom adjacent to the carbene carbon, the carbene is a triplet
¶ Bonding between Carbene and Metal Centre
The two types of carbene have different reactivity
- In other words, the nature of the metal-carbene bond is different depending on the electron configuration of the carbene
¶ Singlet Carbene
- The interaction happens due to electron donation from the sp2 orbital of carbene to one of the orbitals in the metal
- The interaction happens due to electron donation from one of the orbitals in the metal to the empty p orbital of carbene
¶ Triplet Carbene
- The interaction happens due to electron donation from the sp2 orbital of carbene to one of the orbitals in the metal
- The donation happens due to electron donation from the partially filled p orbital of carbene to one of the orbitals in the metal
In general, we can summarize the difference in bonding as such
Singlet Carbene are L type ligands
Triplet Carbene are X2 type ligands
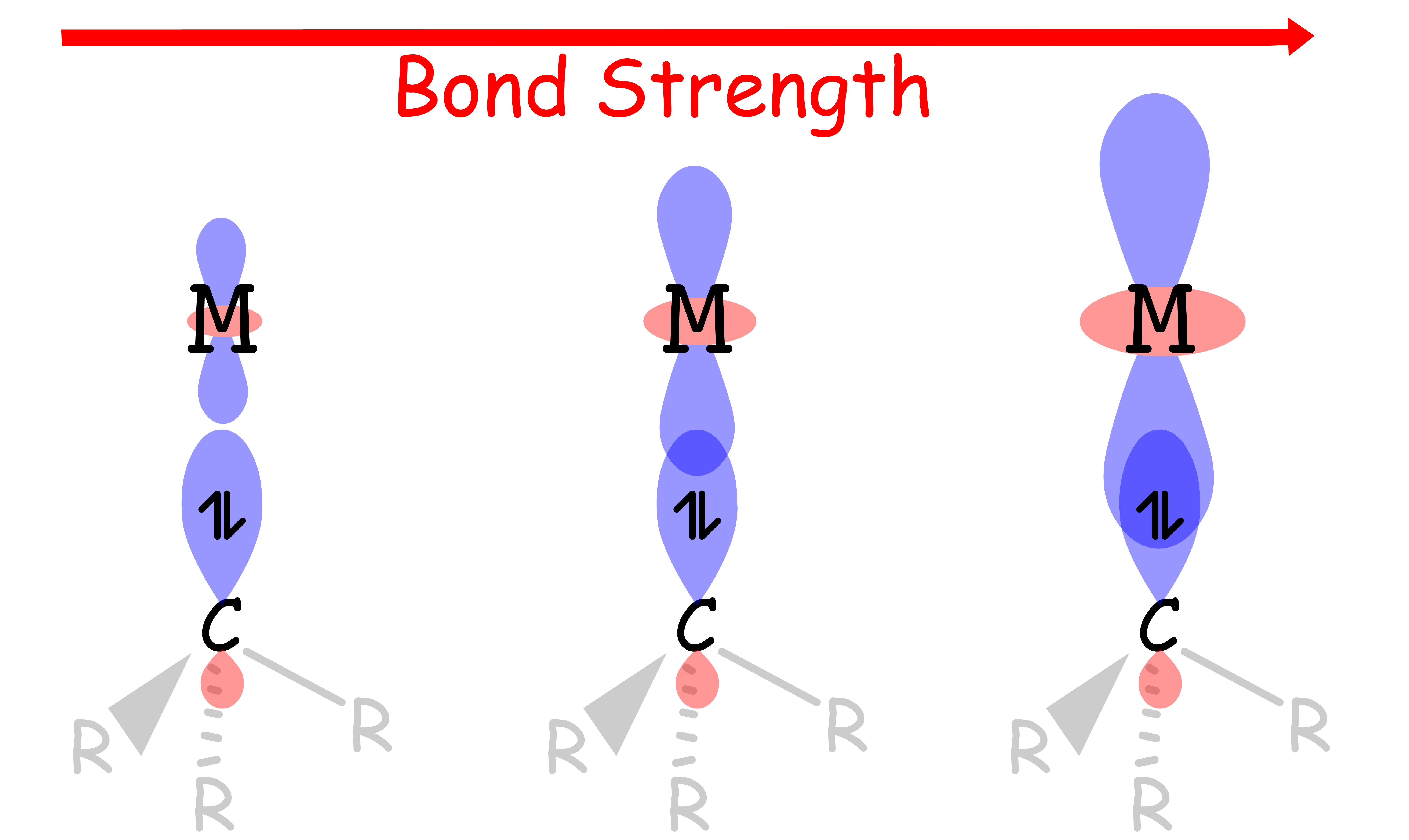
¶ Transition Metal Alkyls
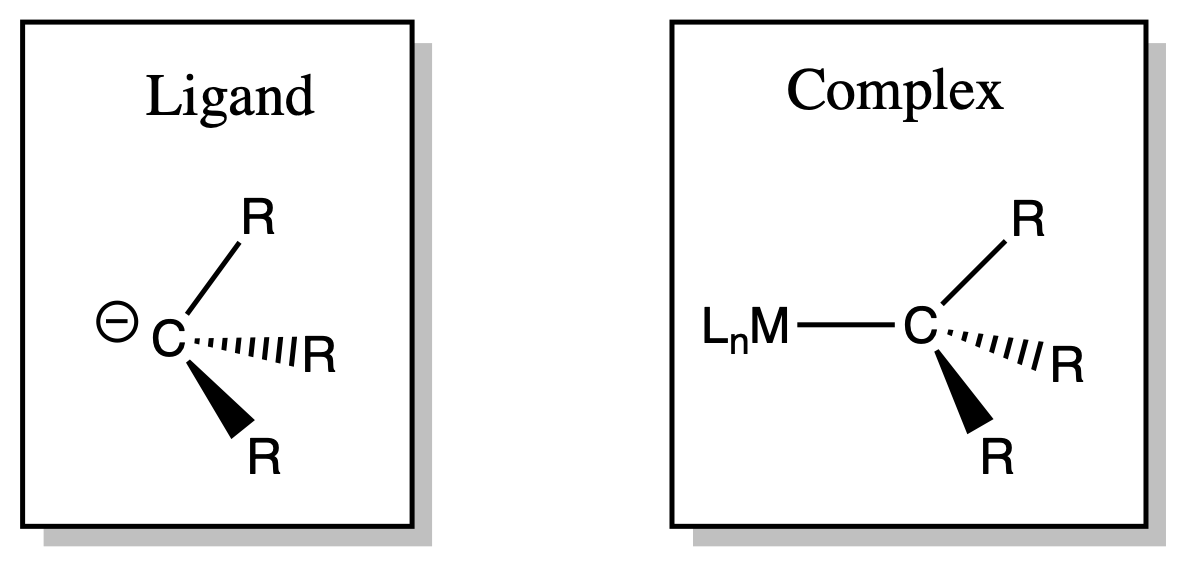
Alkyl ligands are X-type ligands
- Alkyl ligands are strong -donor
- The groups can be functional groups ( e.g. hydroxyl, halide, ester e.t.c. )
The metal–alkyl bond strength is determined by two factors
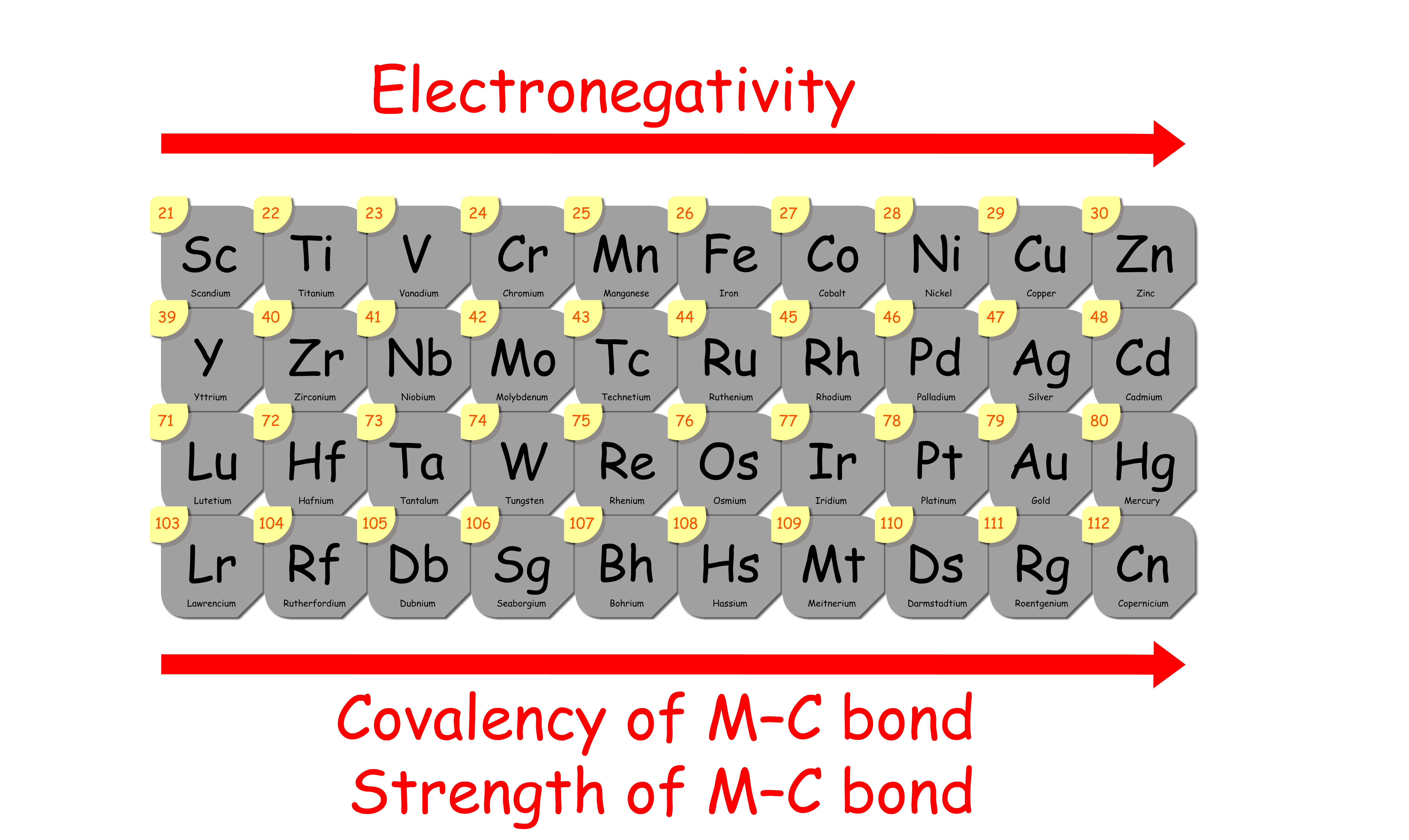
1. Electronegativity
- As we go across the d-block, the electronegativity of the transition metals increases
- This means that the metal–alkyl bond becomes more covalent as we go across the the d-block
- In other words, the metal–alkyl bond strength increases from left to right
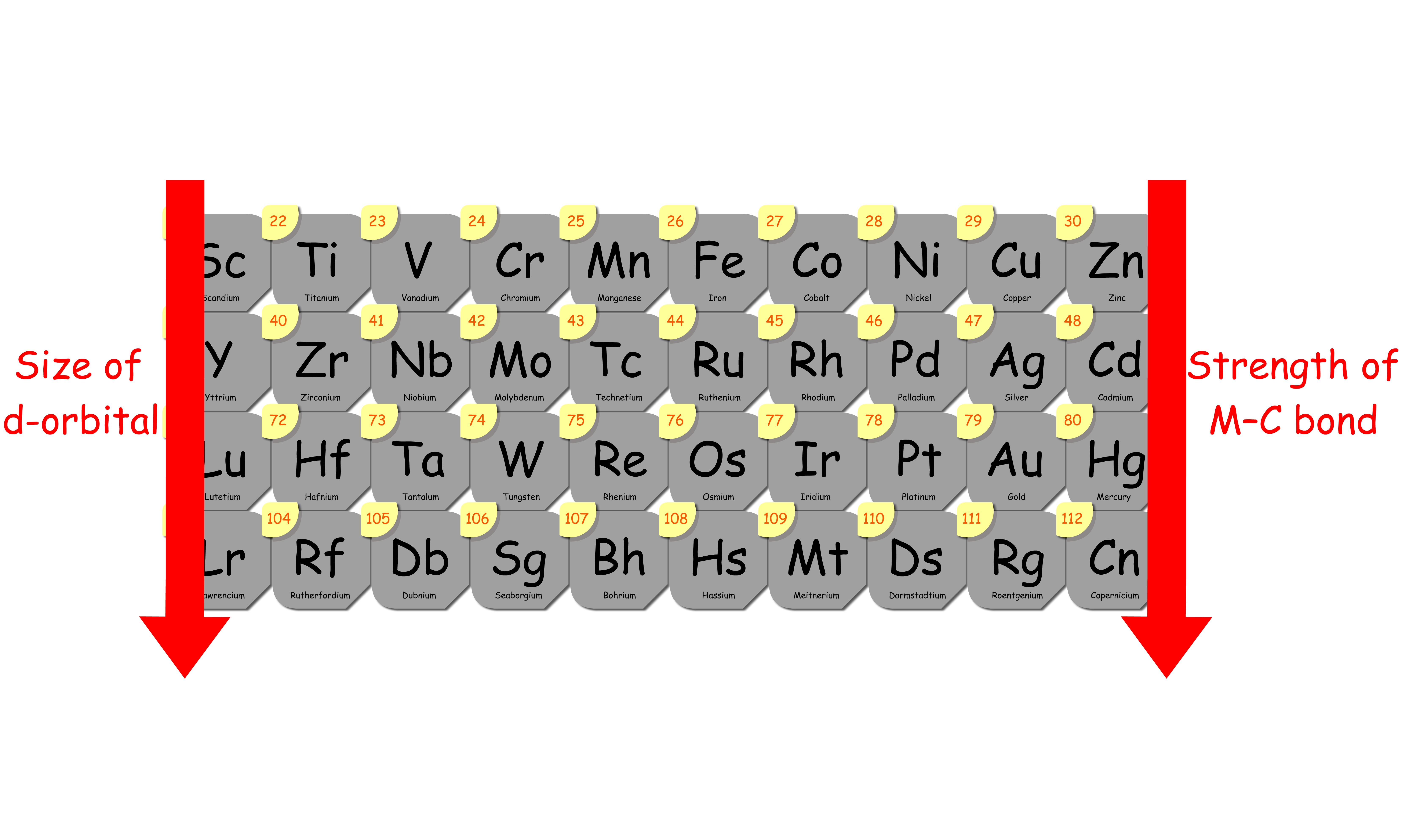
2. Orbital Overlap
- Bond strength increases as the overlap between the sp3 orbital of the alkyl carbon and the d-orbital of metal increases
- The key factor that dictates how well the overlap is depends on the size of the d-orbital
- The size of the d-orbital increases down the group, so the overlap between the orbitals becomes more effective as we travel down the group
- In other words, the metal–alkyl bond strength increases down the group
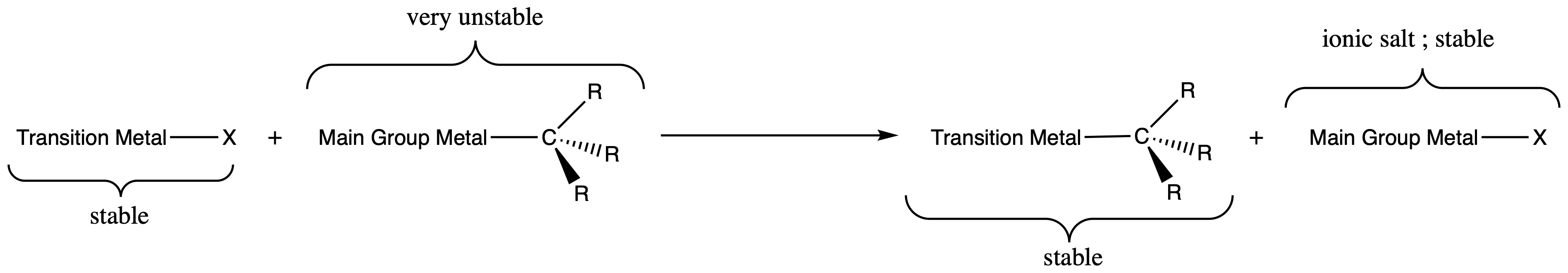
¶ Synthesis of Transition Metal-Alkyls
¶ Transmetallation
- using 'polar’ organometallics ( e.g. R–Li, R–MgX )
- Works when R transferred from more electropositive metal to lesser one
- Thermodynamically () and kinetically (salt precipitation) favorable
- Organometallics are very reactive and it is difficult to only add one alkyl group to the transition metal
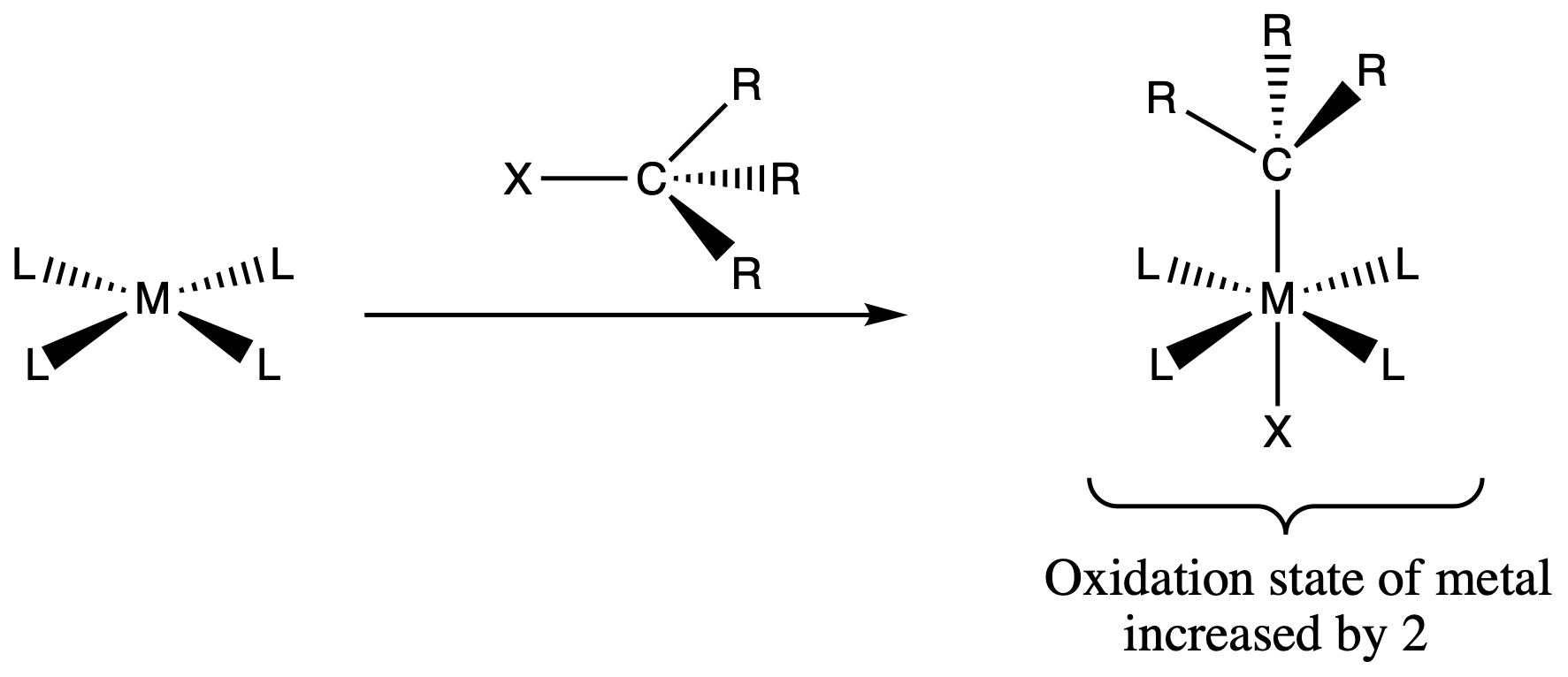
¶ Oxidative Addition
- The complex will go from a 16e- square planar complexes to a 18e- octahedral complex
¶ Alkylation by R+
- The alkylation step is thermodynamically () and kinetically (salt precipitation) favorable
¶ Reactivity of Transition Metal-Alkyls
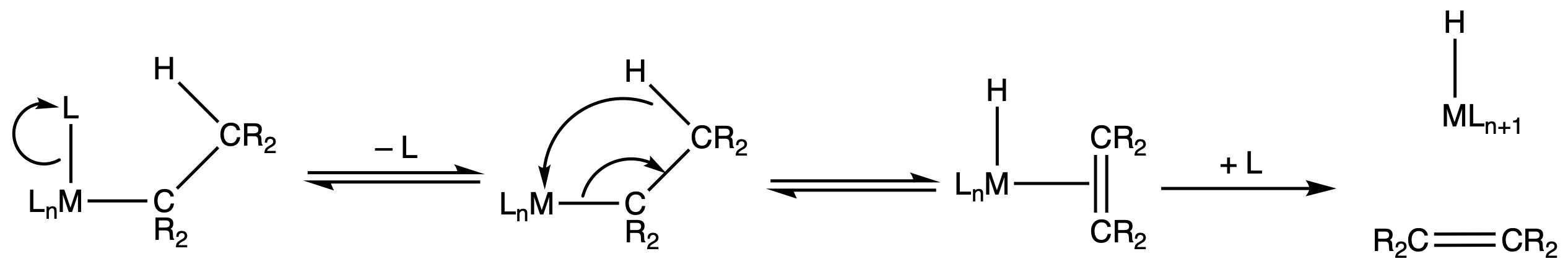
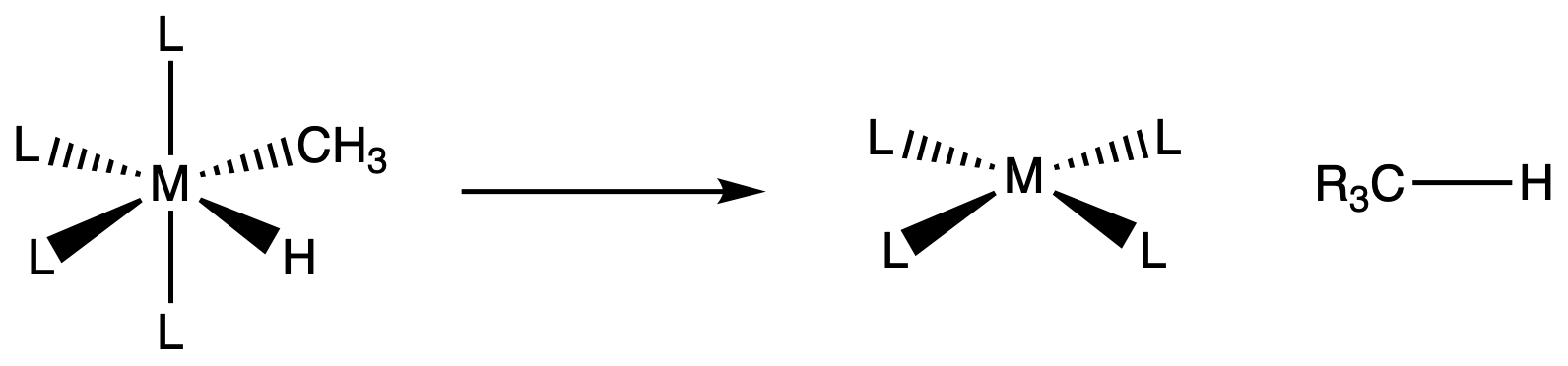
¶ β – hydride elimination
General requirements for β – elimination:
- availability of a planar M-C-C-H arrangement
- β – hydrogen on M-R
- free coordination site ( usually less than 18e– )
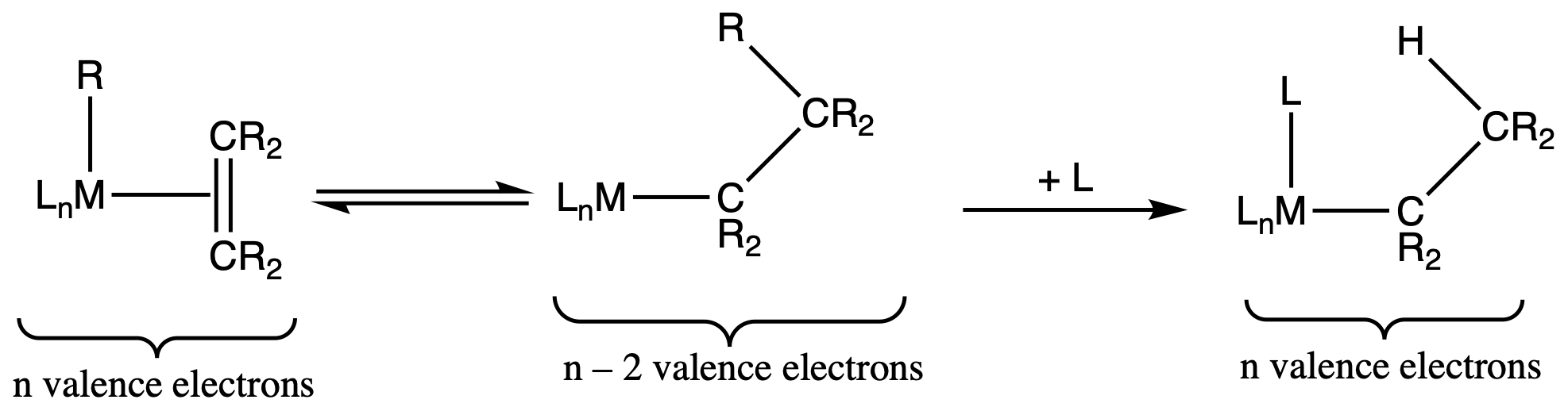
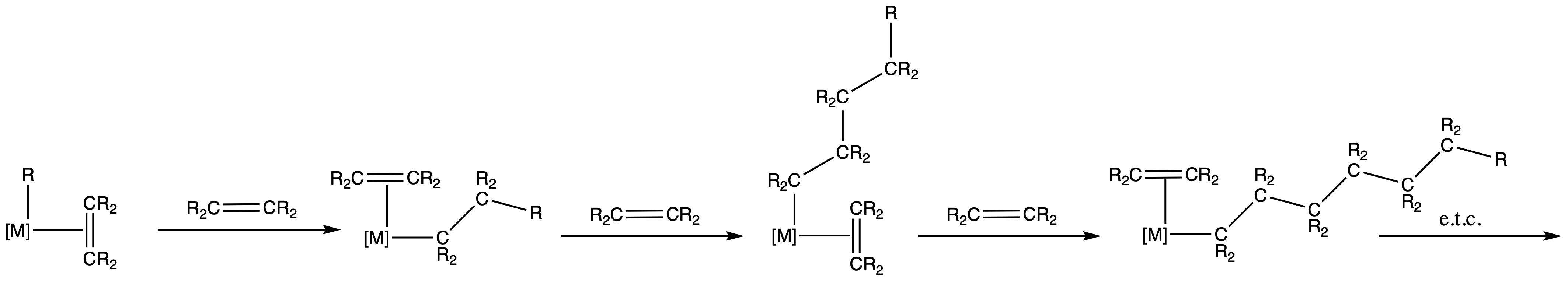
¶ Migratory Insertion
- Migratory insertion is like the reverse of β – elimination
- If L = CR2=CR2, then this reaction is a catalytic polymerisation
¶ Reductive Elimination
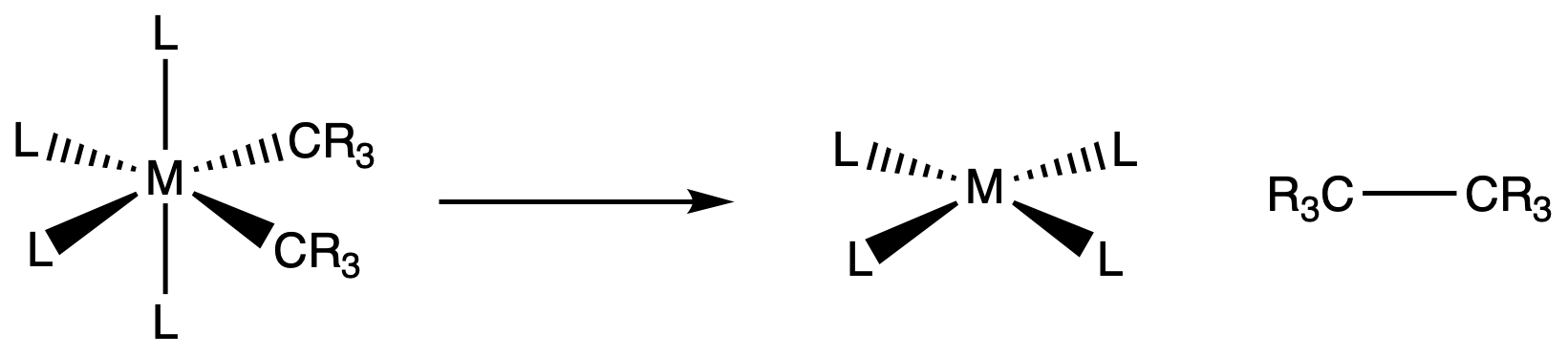
The reaction can take place if a complex has
- 2 -bound cis alkyl ligands
or
- 1 -bound alkyl and a cis hydride
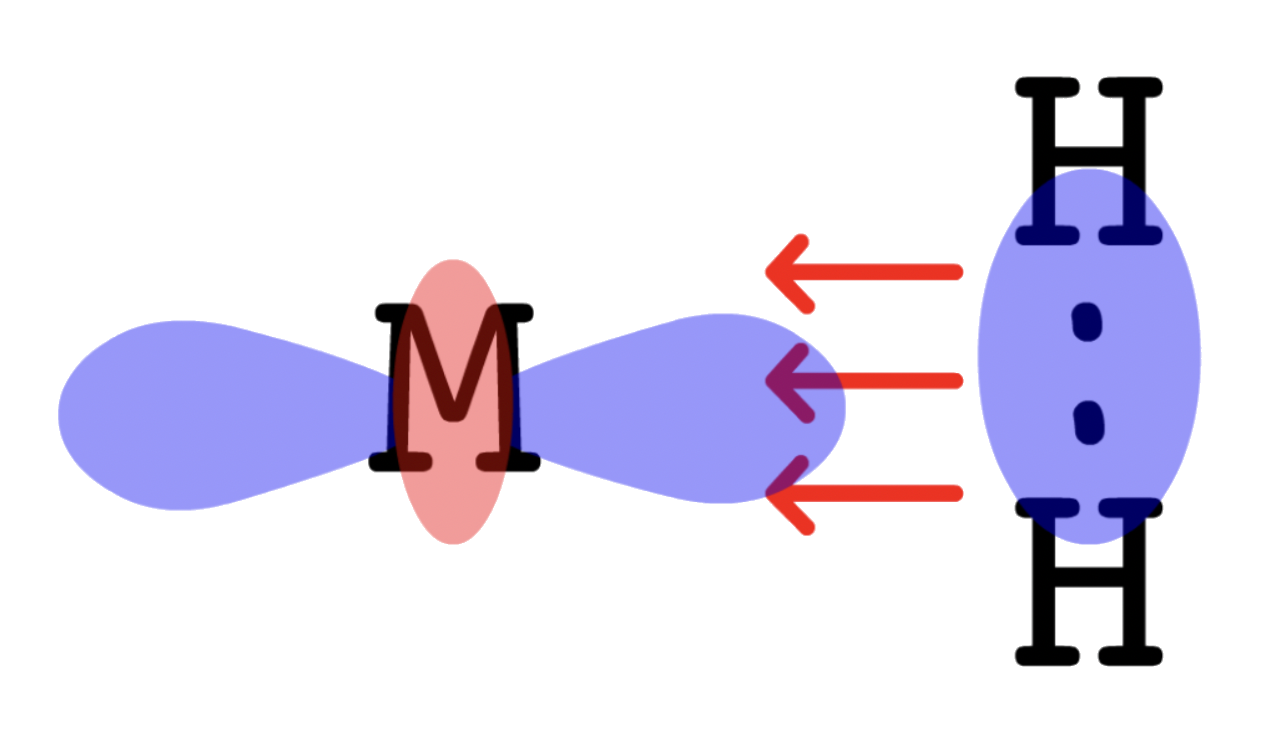
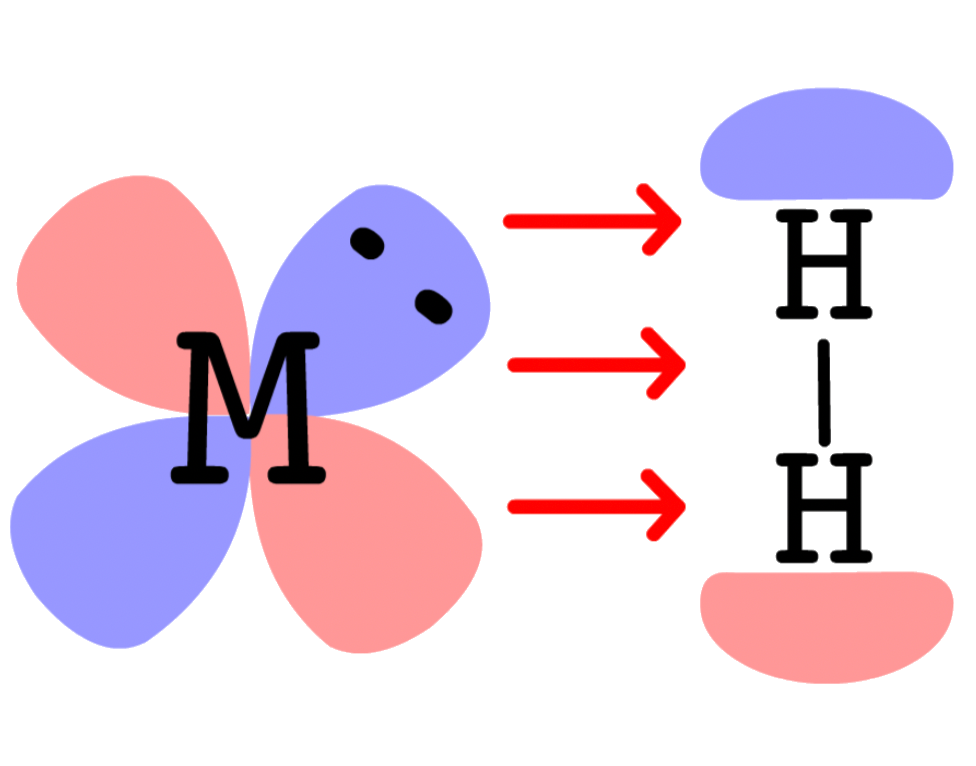
¶ Metal Dihydrogen Complexes
The formation of transition metal complex of dihydrogen is extremely important in understanding a lot of catalytic reactions
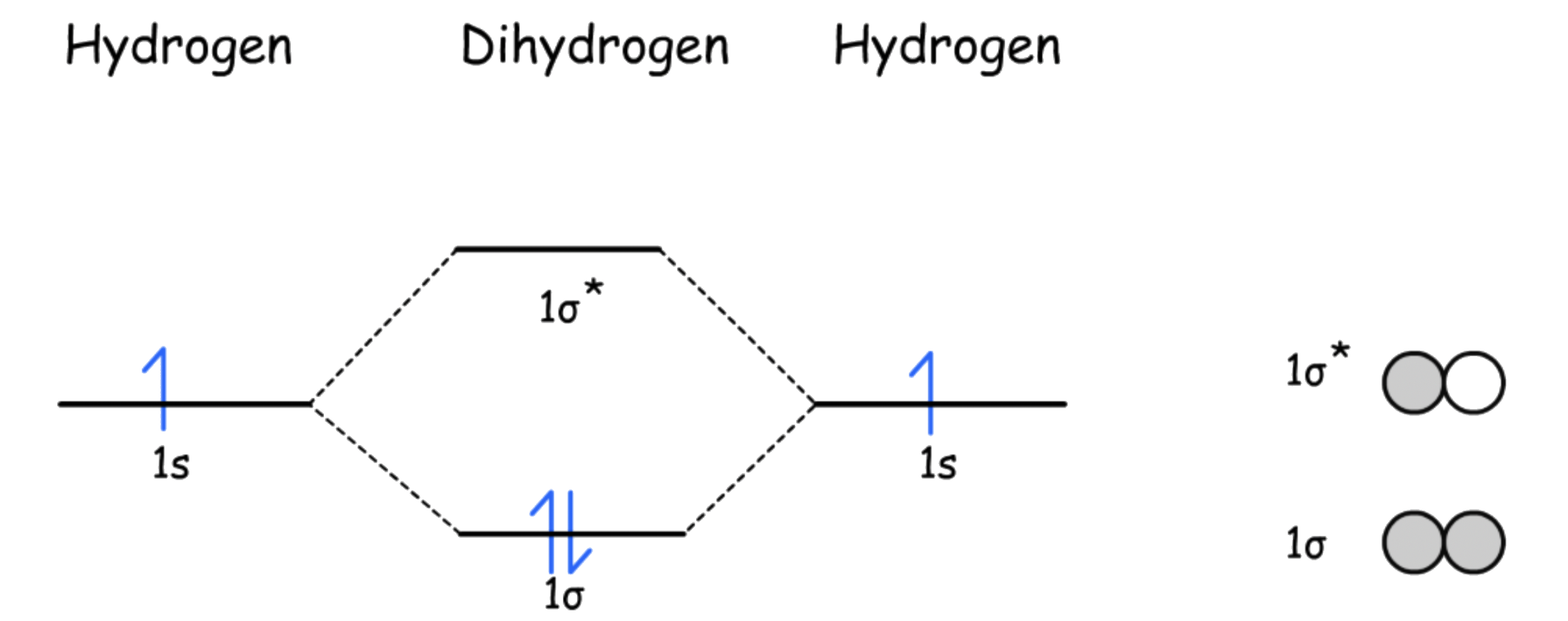
The molecular orbital diagram of H2 is given as such
- The HOMO of H2 is , so the electron pair will be donated from to a vacant orbital on M ( interactions )
- The LUMO of H2 is , so the electron pair will go from the filled orbital of M back to of H2 ( interactions )
- The sigma and pi interactions require the H–H bond to be perpendicular to the M–H2 bond
The π-back-bonding weakens the H-H bond for two reasons
- The HOMO of the H-H is a bonding orbital, the donation of electron pair from the bonding orbital will lower its bond order, thus weakening the CO bond
- The LUMO of the ligand is an antibonding orbital, the acceptance of electrons will lower the bond order, thus weakening the H-H bond
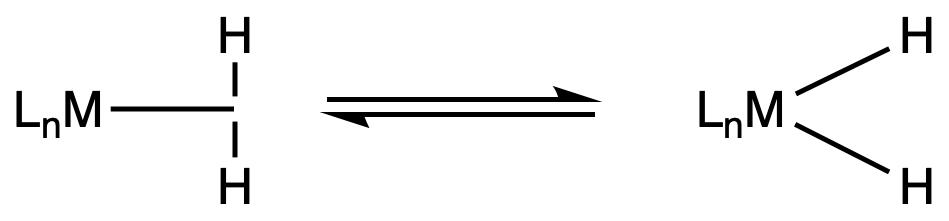
Depending on the strength of the back-bonding, the H2 can be split into two hydrides
- The ligand type changes from ( Dihydrogen ) to ( 2 Hydrides )
The strength of the back-bonding depends on the transition metal
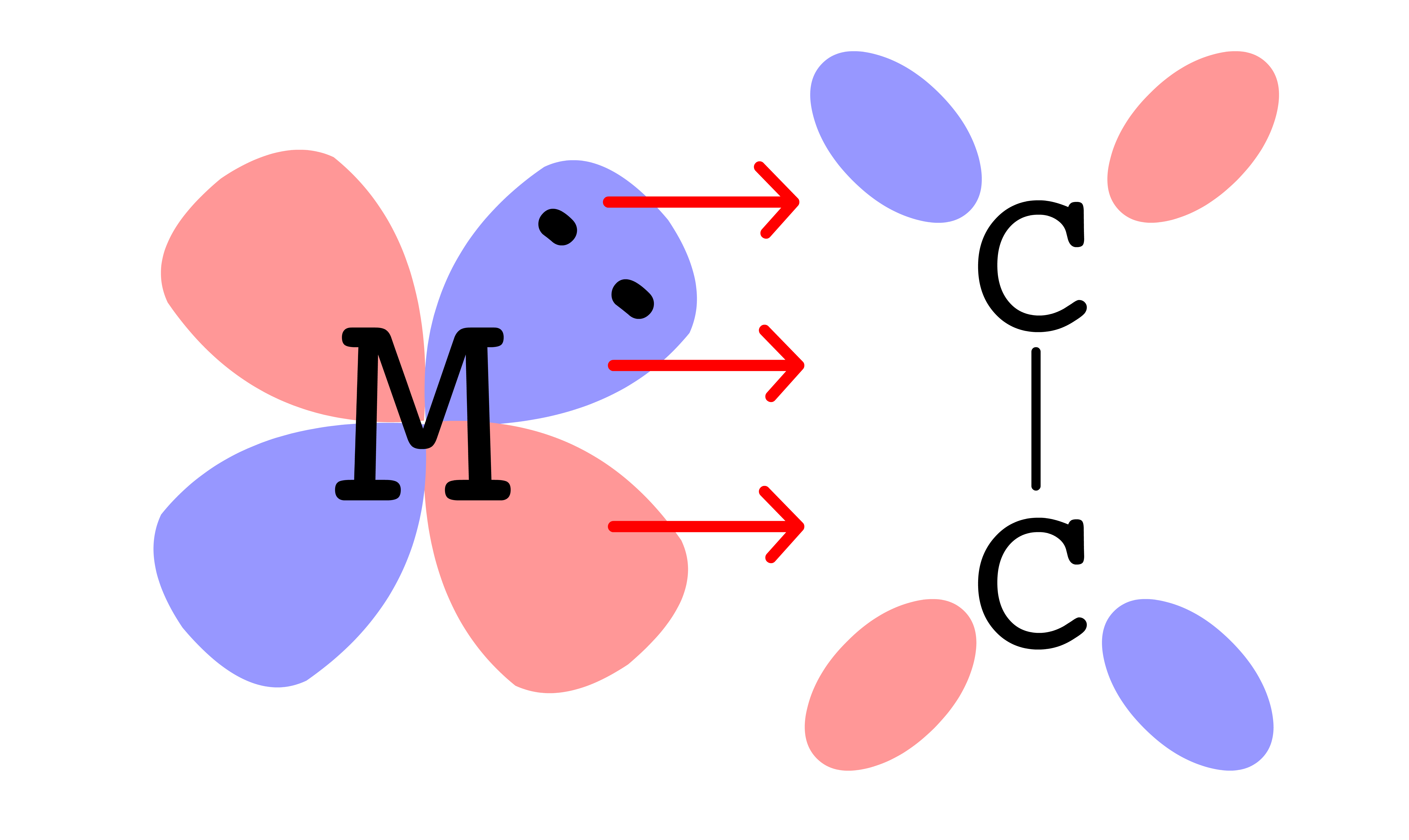
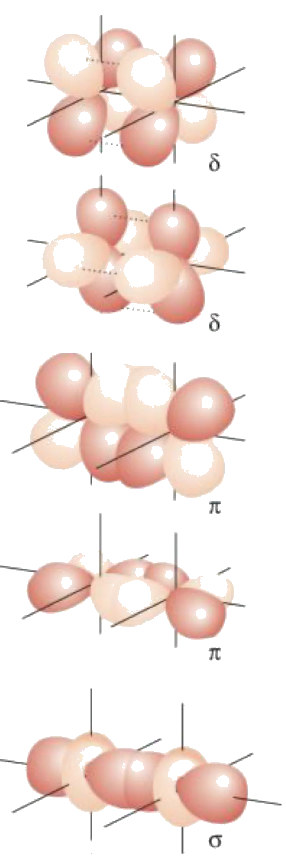
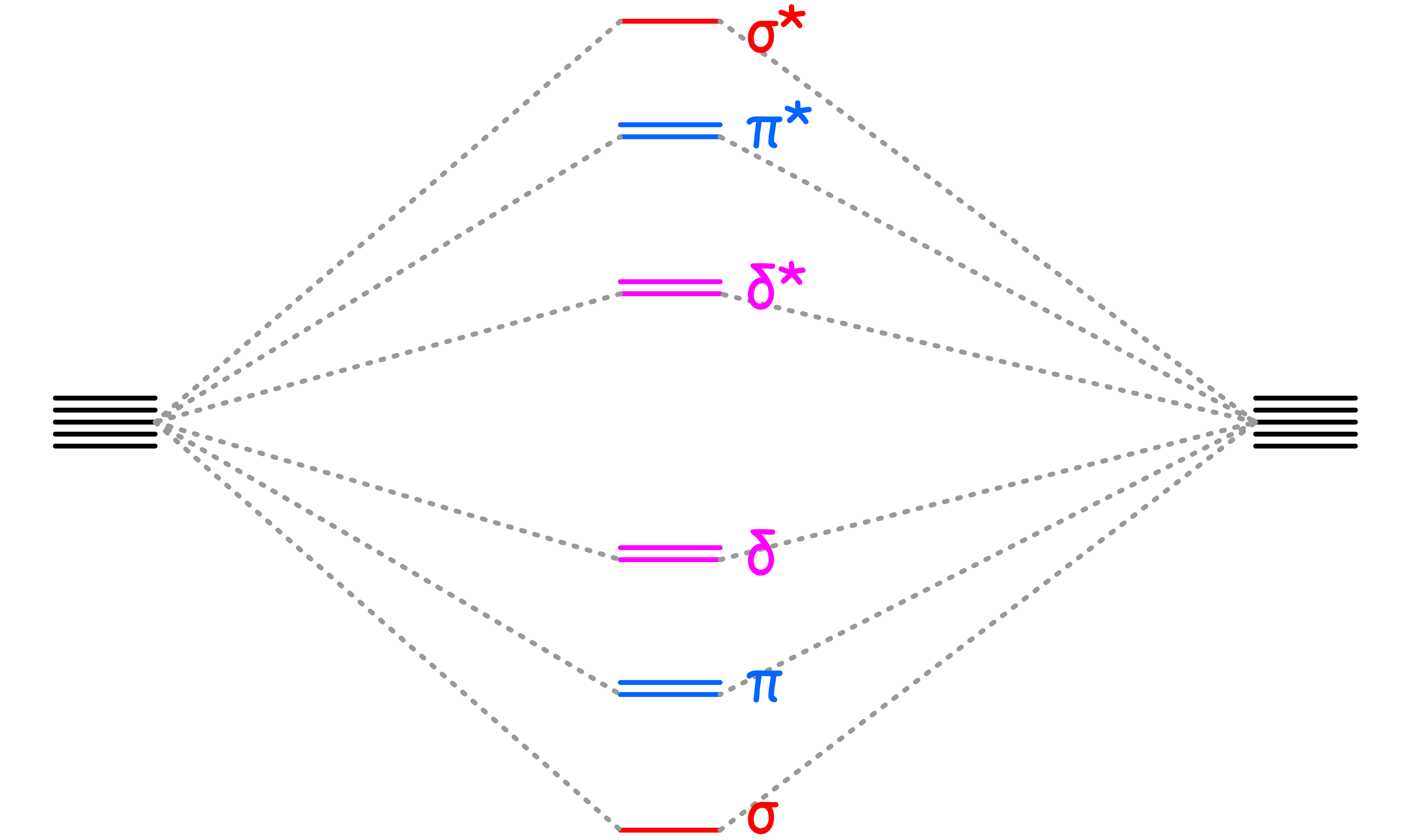
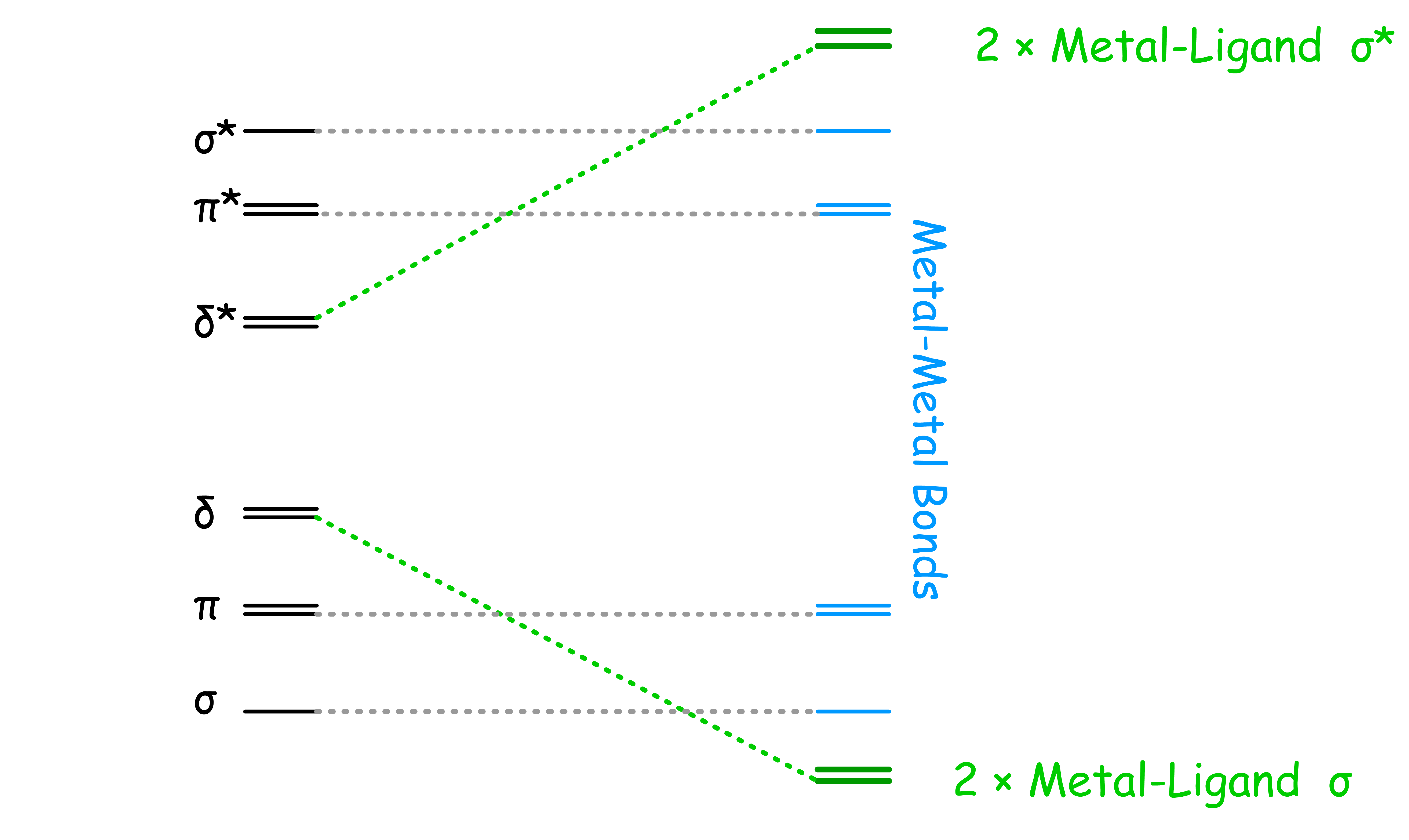
¶ Metal-Metal Multiple Bonding
Metals are capable of forming multiple bonds with each other
Metals can form at most five bonds with each other: , and bonds
- The splitting goes down from to to
- In the best case scenario, all 5 of the bonding orbitals between the metals are filled, resulting in a quintuple bond. However, due to bonding between metal and ligands, quintuble bond is rarely achieved
¶ Quadruple and Triple bonds
¶ Quadruple Bonds
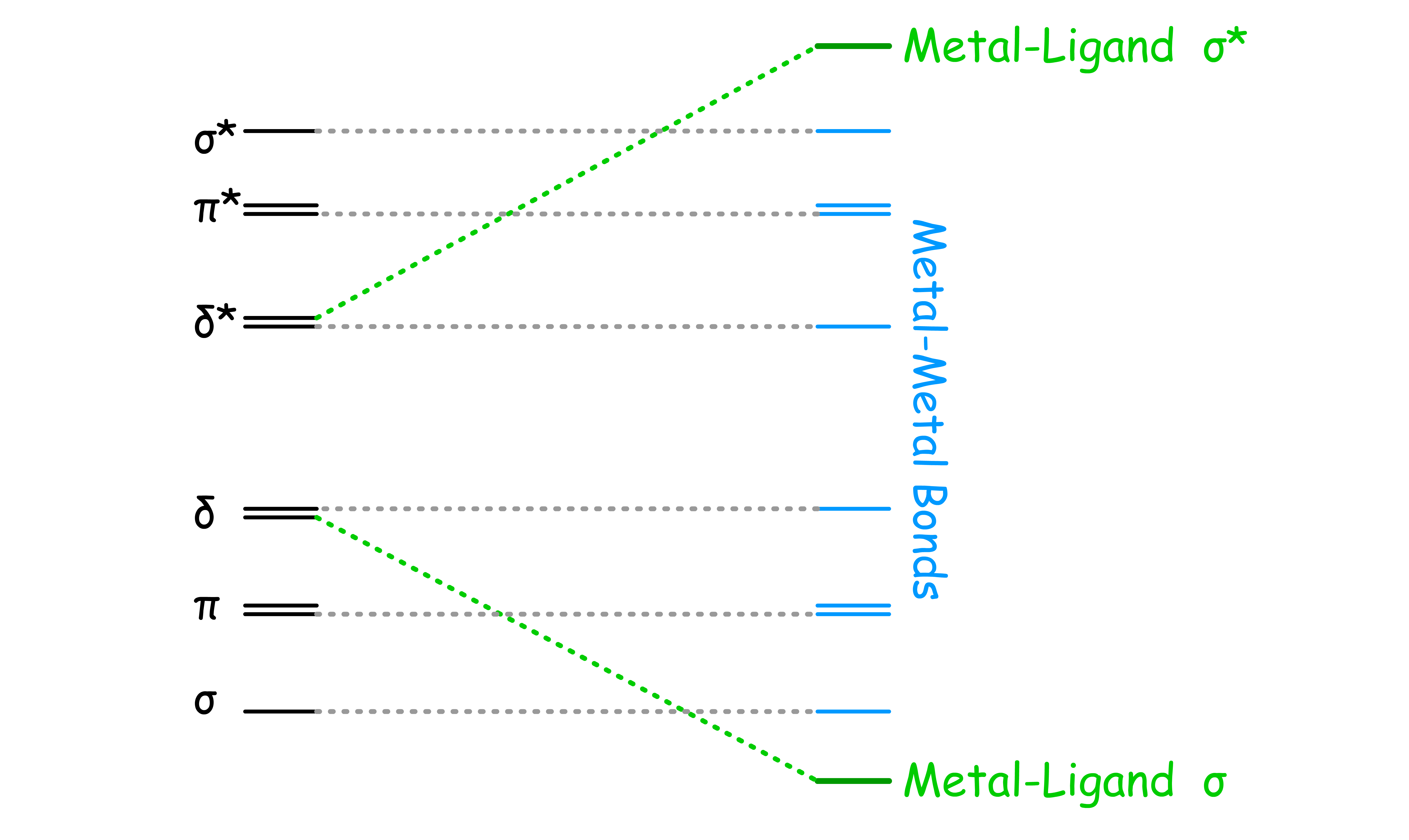
Quadruple bonds happens when one of the d-orbitals that is originally involved in bonding is used to form metal-ligand bond
- Involvement of the d orbital with the ligands effectively removes it from participating in M-M bonding
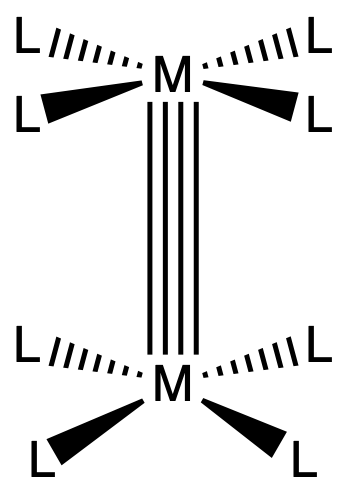
Complexes with quadruple bonds have eclipsed D4h-like ligand conformations
- There is clear steric repulsion between the ligands that could be relieved by rotation to a staggered geometry
- However, the -bond component of the quadruple bond favors an eclipsed orientation and imposes a rotational barrier for rotations about the quadruple bond
¶ Triple Bonds
Triple bonds happens when both of the d-orbital that are originally involved in bonding are used to form metal-ligand bond
- Involvement of the d orbital with the ligands effectively removes them from participating in M-M bonding
- M2L6 in the eclipsed conformation gives best overlap, however, most compounds of this type are in fact staggered due to steric reasons