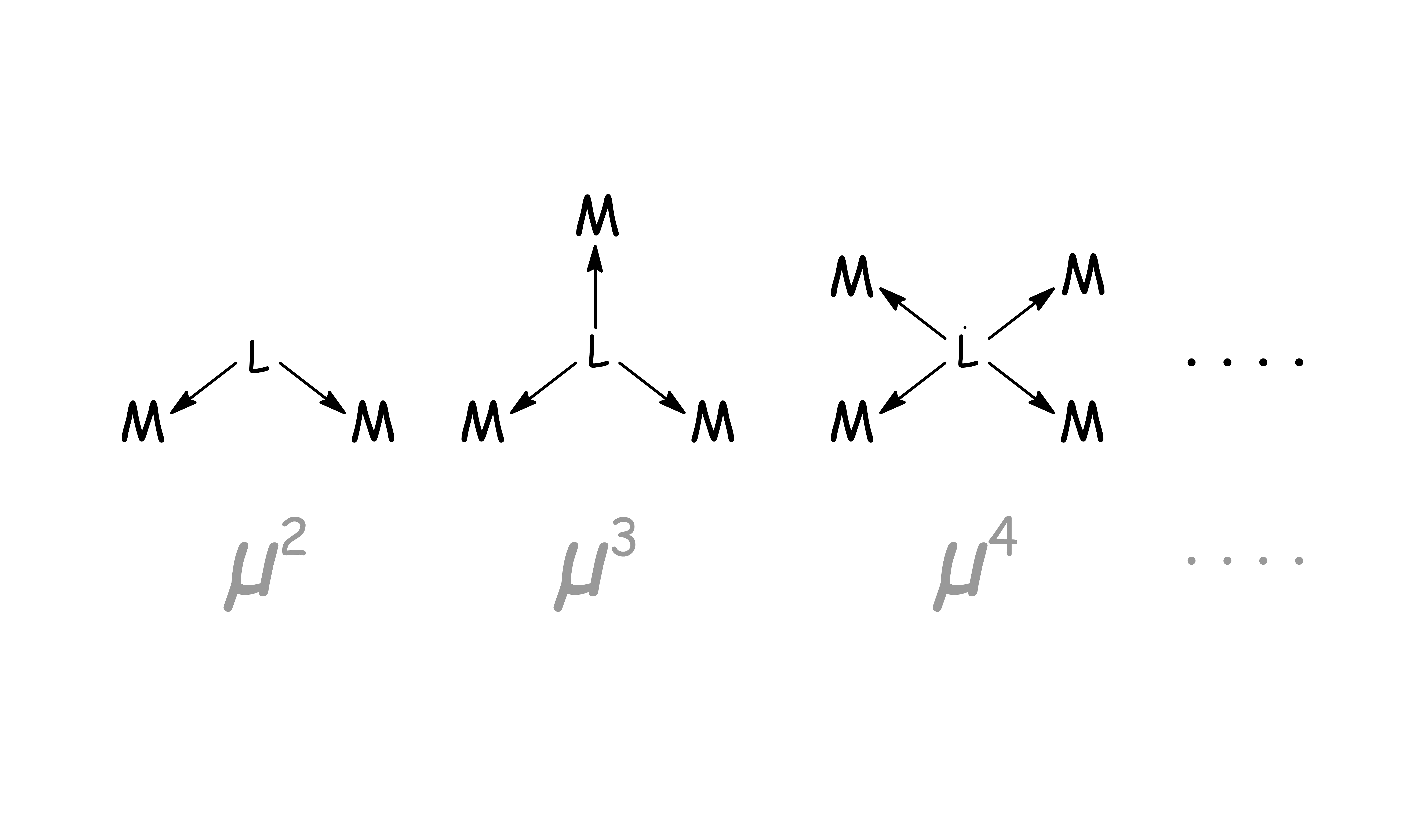¶ Electron Configuration of Transition Metals
In previous section, we introduced the electronic structure of transition metals by considering them as gaseous neutral atoms
- This leads to confusion concerning the occupancy of d-orbitals in compounds of the transition elements and the consequent assignment of dn values
In gaseous neutral atom, the ( n + 1 ) s-orbital is more stable ( lower in energy ) than n d-orbital
- However, the electronic configurations of transition-metal cations tend to revert to a hydrogen-like order
- For cations, the n d-level is lower in energy than the ( n + 1) s-level, which in turns is more stable than the ( n + 1 ) p-level
When we are dealing with transition metal compounds, the metal's valence-shell electrons are treated as if they are all in the n d-orbital
- In coordination compounds, the d-orbital is stabilized to a much greater degree, so 4s, 5s and 6s orbitals are not occupied
- This convention is a fair description of reality of transition metals in higher oxidation states and for those elements to the right of the transition series
- In neutral complexes of the early transition metals, it is a less-good assumption
Within compounds of the entire transition series, the d levels are those which have the highest energy and are consequently those which electrons can be most easily removed by oxidation or added to by reduction
- Thus, we shall see that d-electron levels, referred to as dn, are primarily associated with the metal, and these metallic d-electrons can be considered as non-bonding
- Except for small perturbations brought about by π-bonding effects and orbital splitting through interaction with the σ-bonds of the ligand groups
It should be apparent that a relationship is expected between the d-electron configuration ( dn ) and the oxidation state
- This is because it is the level which electrons are added to or removed to
¶ 18-Electron Rule
Lewis dot diagrams are not useful for transition metal complexes since these metals do not obey the octet rule and also have overlapping energy sublevels
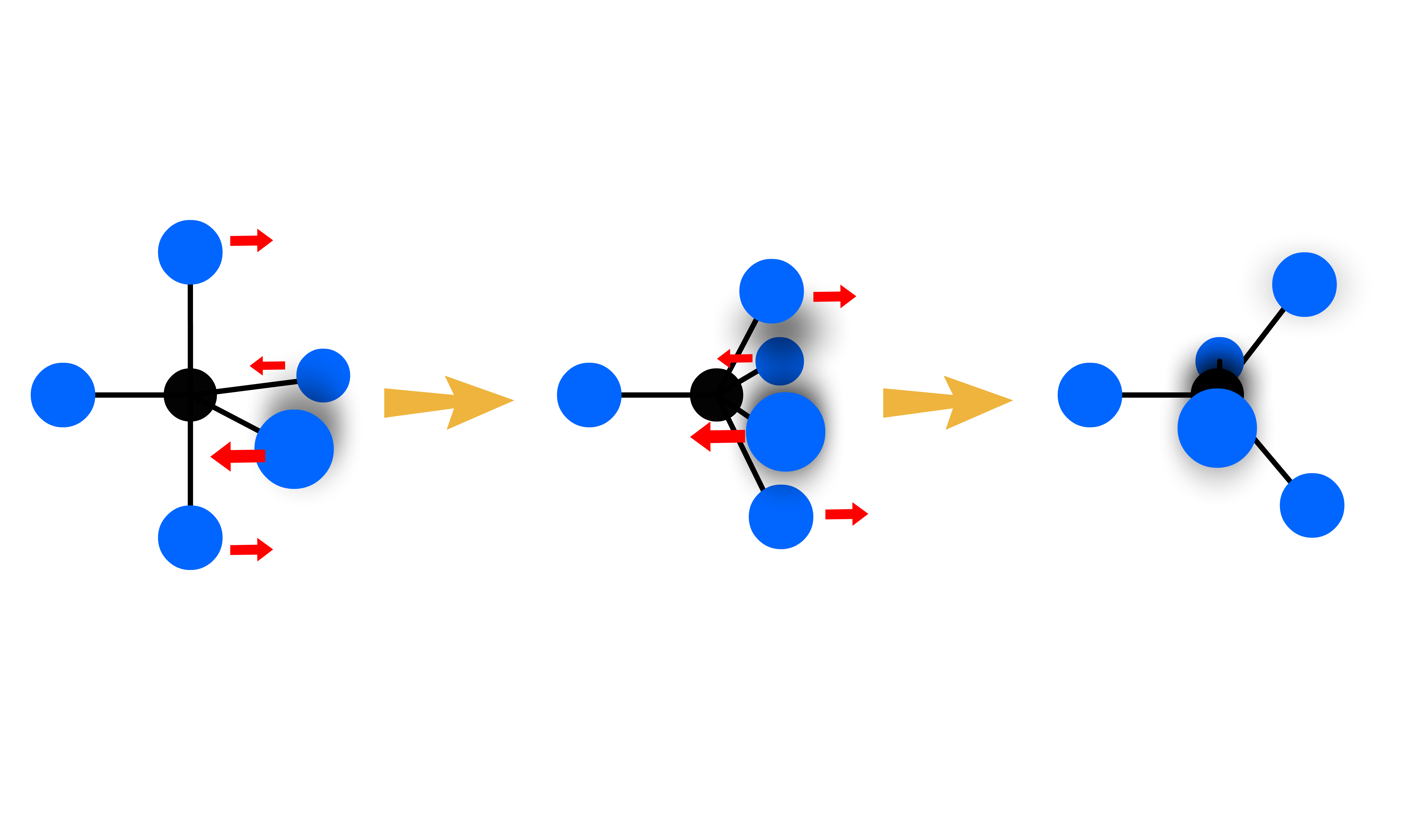
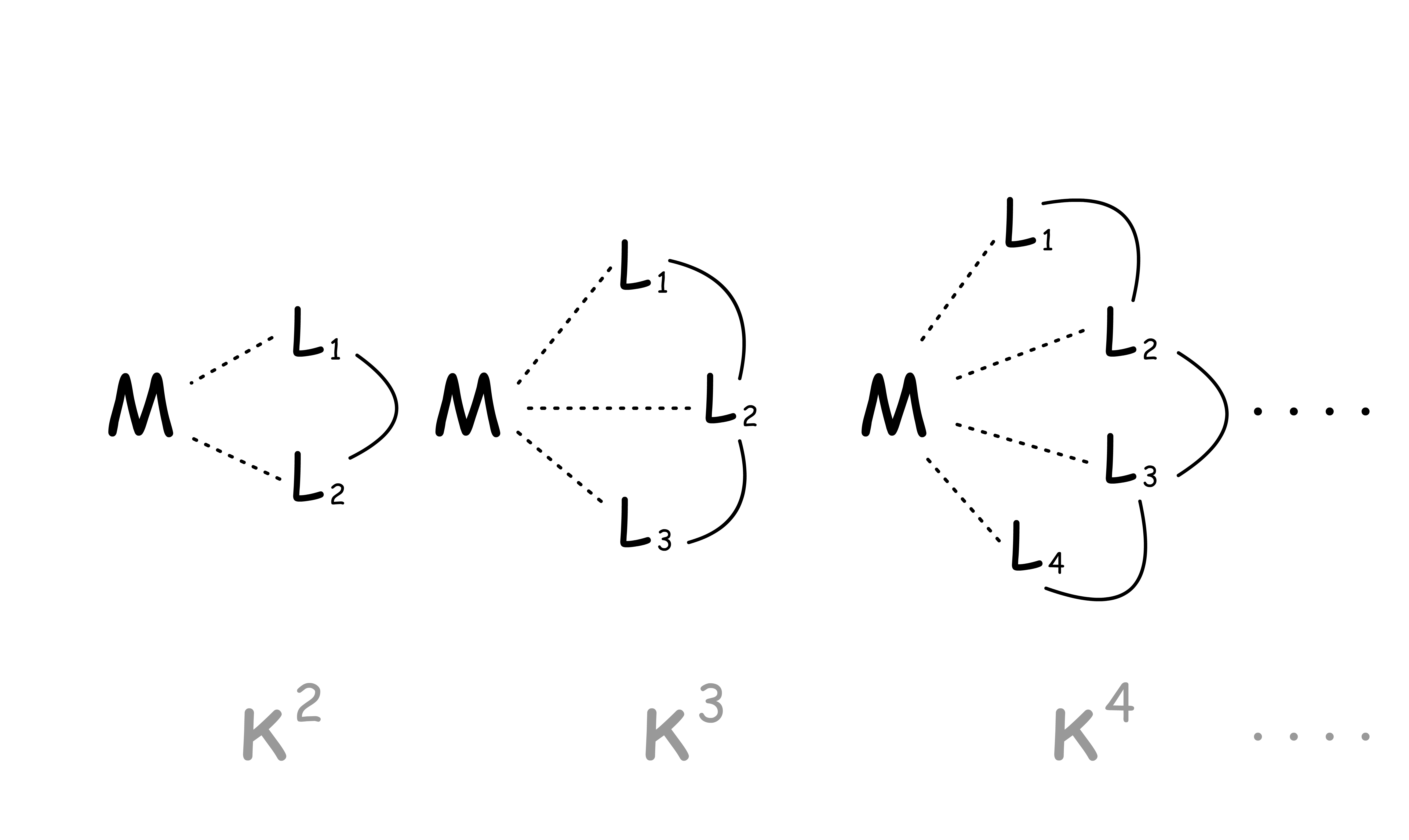
- Coordination number is defined as the number of ligands immediately surrounding a central atom / ion in a complex or crystal
There is a maximum coordination number ( CNmax ) permitted for each dn, provided that
- We only consider monometallic, diamagnetic compounds
- n is an even number and all the electrons are paired
- This relationship arises from the unoccupied s-, p- and d-orbitals which are conceptually used to fashion molecular orbitals to accommodate ligands
The relation between CNmax and dn is:
- Rearranging the expression, we get:
- There is an inverse relationship between the number of non-bonding, metal-centered d-valence electrons and the maximum number of ligands which can be combined with the metal, provided that the electrons are spin-paired
- This is a logical assumption since these non-bonding, metal-localized d-electrons have their principle electron density on the metal and in between ligands
The numerical relationship between CNmax and dn is often referred to as the 18-electrons rule
- The 18 electrons rule states that it is thermodynamically stable for transition metals to have 18 valence electrons
- The 18 electron rule has very few exceptions among well-characterized, stable organometallic or coordination compounds of the transition metals, provided that it follows the above assumptions
- This rule is not helpful when considering the complexes of non-transition metals
¶ Kepert Model
The VSEPR model cannot be applied successfully to transition metal complexes
- The VSEPR model takes non-bonding electrons in to account when considering the structure of molecules
In the Kepert Model, only the ligands, but not the non-bonding electrons on the transition metal, are taken into account when thinking about their structures
- Complexes with different electron counts can still adopt the same shape. In other words, the geometry is independent of the electron configuration
- The number and types of ligands is the main contributing factor to the complex's geometry
The Kepert Model rationalizes the shapes of a d block metal complex [ MLn ], [ MLn ]m+, [ MLn ]m- base on the number of ligands ( n )
Coordination Number = 1 : Linear
- Requires extremely sterically bulky ligands such that it hinders other ligands from binding to the metal center
- Rare
Coordination Number = 2 : Linear
- Common for metals of Group 11 and 12
Coordination Number = 3 : Trigonal Planar
- Usually involves sterically bulky ligands
- Quite rare
Coordination Number = 4 : Tetrahedral
- The geometry is favored over higher CN if the central atom is small or if the ligands are large
- Metal complexes from d0 to d10 are all able to adopts this geometry
- Common for oxoanions of metal atoms in high oxidation states and for halide complexes of M2+ in the first row of d-block
Coordination Number = 4 : Square Planar
- Less common than tetrahedral
- Mainly associated with d8 metal ions
Coordination Number = 5 : Trigonal Bipyramidal and Square-Based Pyramid
- Both geometries are quite commonly adopted, even by the same compound
- The energy difference between the two geometries is very small
Complexes that adopt these structures can undergo Berry Pseudorotation:
Coordination Number = 6 : Octahedral
- This geometry is extremely important amongst all d1 to d9 complexes
- Extremely common
- Distortion for MX5Y, MX4Y2 or MX3Y3 are very common
Coordination Number = 6 : Trigonal Prismatic
- Extremely rare
- Often d0 complexes of ML6
¶ Ligands
Transition metals form coordination compounds, consisting of a metal bonding to molecules or ions ( referred to as ligands )
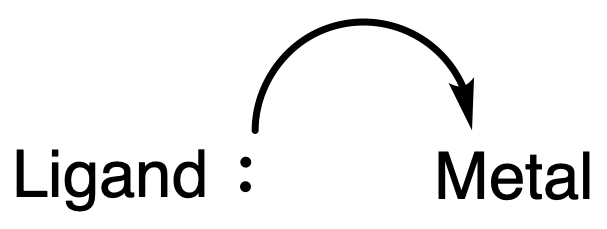
- The ligands are attached to the central atom by dative bonds, also known as coordinate bonds, in which both electrons in the bond are supplied by the same atom on the ligand
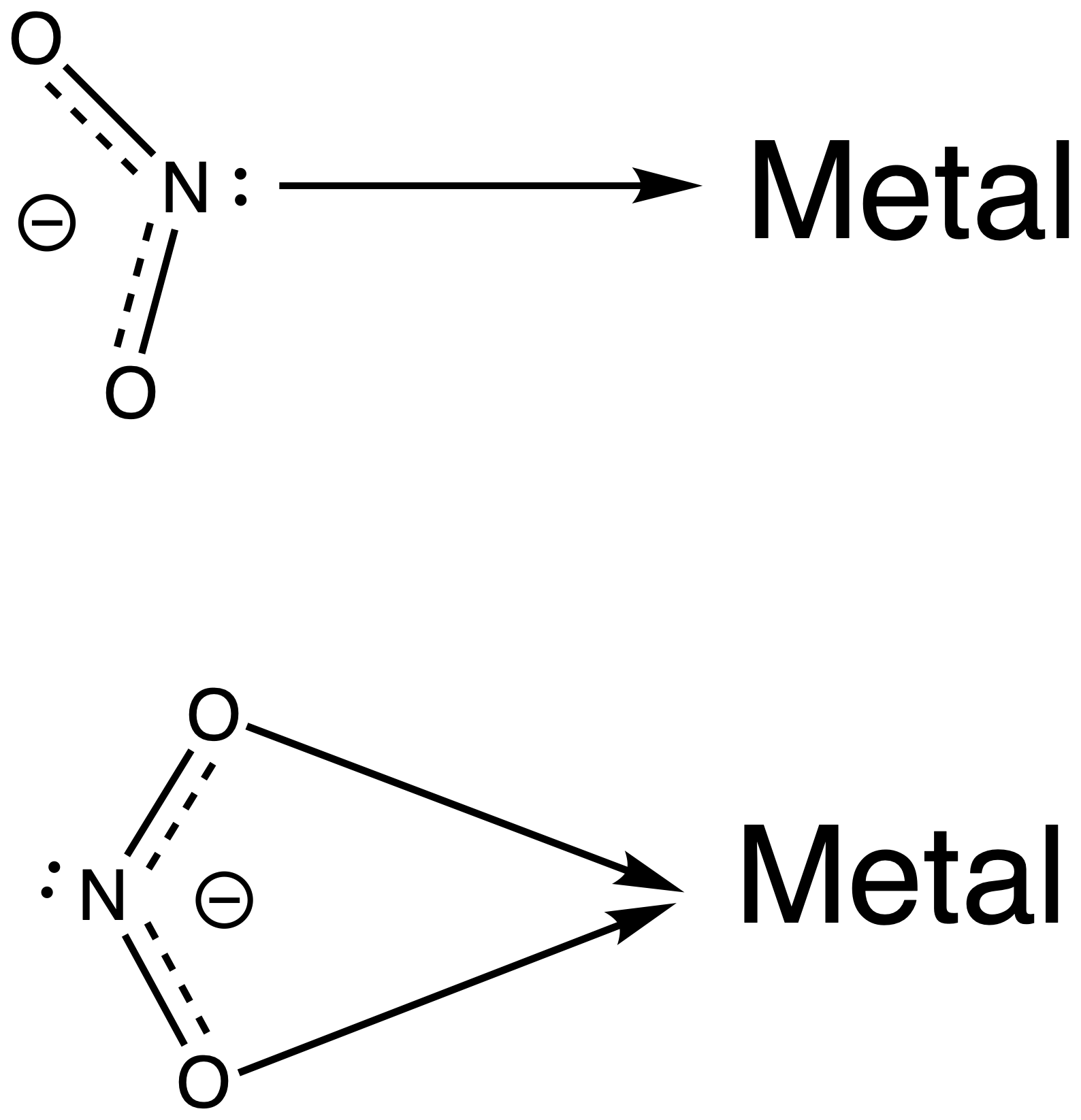
- Ligands usually act as Lewis bases or nucleophiles, donating a pair of electrons to form the ligand-metal bonds ( Coordination Bonds )
- Ligands will donate electron density to the metal center in a dative manner, so the coordination bond should be represented using an arrow:
- However, often it is assumed that the nature of the bond is clear and just a single line is used
- Since transition metals have access to the d-orbitals, they have a lot of unique properties that cannot be explained by simple models ( like Lewis dot diagrams, VSEPR )
¶ Synergic Bonding
Ligands must possess the ability to donate electrons, but it can also have the ability to accept back the electron density
- Donor: donate a pair of electrons from their HOMO to an empty orbital that can have sigma interaction on the metal to form a 𝜎–bonding interaction
- Acceptor: donate a pair of electrons from their HOMO to an empty orbital that can have sigma interaction on the metal to form a 𝜎–bonding interaction
There are 4 ways a ligand can interact with the metal center and a ligand can interact with the metall center in more than one way:
- – donor ligand
- Donating electron density using s-orbital or spn-hybrid orbital
- Donate a pair of electrons from their HOMO to an empty orbital that can have sigma interaction on the metal to form a –bonding interaction
- – acceptor ligand
- Accepting electron density using s-orbitals
- Their LUMO is an orbital capable of forming bonds
- Extremely rare
- – donor ligand
- Donating electron density using p-orbitals
- Pi donor ligands are those which have extra non-bonding electrons in their valence orbitals
- Their HOMO is an orbital capable of forming bonds
- – acceptor ligand
- Accepting electron density using p-orbitals
- Their LUMO is an orbital capable of forming bond
Donation of electron pair to the metal center greatly increases the negative charge at the metal
- According to the Pauling's electroneutrality principal, the distribution of charge in a molecule or ion is such that the charge on any single atom is ideally close to zero. Hence, it is not favored for the metal center to bear a high negative charge
- If the ligand can act as a acceptor, then the electron density can be transferred back from the metal center to the ligands
These effects reinforce each other via a push-and-pull mechanism
- The better the –donation, the more negatively charged the metal center would be, the stronger the back donation
- This is called the synergic effect ( working together )
Synergic Bonding can stabilize metals in low oxidation states by letting ligands act as acceptor
- Transition metals in low oxidation states are electron rich
Effect of synergic bonding on ligands:
- If the HOMO of the ligand is a bonding orbital, the donation of electron pair from the HOMO will lower its bond order, thus decreasing the bond strength within the ligand
- If the LUMO of the ligand is an antibonding orbital, the acceptance of electrons will lower the bond order, thus decreasing the bond strength within the ligand
- The more negatively charged or electron rich the metal is, the stronger the -back donation
¶ Ligands with multiple electron-donating site
Many ligands are capable of binding metal ions through multiple sites >- This is usually happens when the ligands have lone pairs on more than one atom or the electron density is dispersed through multiple atoms
We can differentiate whether the donors are neighboring or non-neighboring
- Neigboring means that the donating sites are directly bonded together
¶ Hapticity
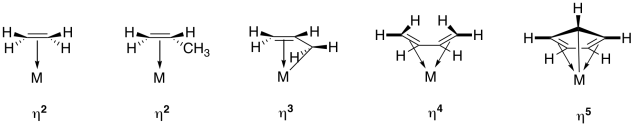
Hapticity is the coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms
- Hapticity refers to the number of neighboring contiguous atoms in a ligand that are coordinated simultaneously to a metal centre
- When they are neighboring donors, the ligand will bind in such a way that all the donating sites are binding equally and have the same distance to the metal centre
The donating sites must be equally bound to the binding site
- Hapticity is counting the number of donating-sites that are equally contributing
- This happens when the donating region on the ligand is in conjugation with each other
- If their contribution is not equal, we will not consider it as hapticity
The number of electron-donating sites, n, involved in the binding is represented by
- The whole ligand is held to the metal at a single binding site despite having multiple donating site on itself
¶ Denticity
Denticity refers to the number of times a single ligand bonds to a metal through noncontiguous (non-neighboring) donor sites
- The donors are joint via a "bridge"
Chelation is a type of bonding of ions and molecules to metal ions
- It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom
- These ligands are called chelants, chelators, chelating agents, or sequestering agents
The number of electron-donating sites, n, involved in the binding is represented by
- Each donating site is binded to an independent binding site on the metal
¶ Bridging Ligands
A bridging ligand is a ligand that connects two or more atoms, usually metal ions
- The ligand does not necessarily have to polyatomic
When naming a complex wherein a single atom bridges two metals, the bridging ligand is preceded by , with a subscript number denoting the number of metals bound to the bridging ligand:
- Ligands that are not bridging are called terminal ligands
¶ Ambidendate Ligands
Ambidentate ligands have two or more donor atoms but only one donor atom is attached to the metal during complex formation
Which atom will act as the donor will depend of the hardness and softness of the metal ligands
- The donor atom which has a softer character will be preferred when binding to a soft cation
- The donor atom which has a hard character will be preferred when binding to a hard cation
¶ Formation Stability
¶ Stability Constant
A stability constant ( ) is an equilibrium constant for the formation of a complex in solution
- It is a measure of the strength of the interaction between the reagents that come together to form the complex
The formation of a complex between a metal ion, M, and a ligand, L, is in fact usually a substitution reaction
- In aqueous solutions, metal ions will be present as aqueous ions, so the reaction for the formation of the first complex could be written as
- Hence, the stability constant can be written as such
- The expression can be greatly simplified by removing those terms which are constant since the number of water molecules attached to each metal ion and the concentration of water in dilute solution is effectively constant
The substitution of ligand does not just happen once, it can happen again and again and form a whole series of equilibrium constants
The overall formation constant
- Usually, it is expressed in logarithmic form
Values of K typically decrease with each successive substitution
- The equilibrium lies progressively in favor of the reactants
- If they do not, it is likely that a change in geometry occurs ( particularly with small metal ions )
¶ Factors affecting complex stability
Ionic size and charge
- Stability of complexes of s-, p- and f-block metal ions of a given charge normally decreases with increasing cation size
- This is a minor contributing factor to transition metal complexes
Hard and soft metal centers and ligands
- Cations ( Lewis acids ) and ligands ( Lewis bases ) were classified as hard and soft
- Hard cations form more stable complexes with hard ligands ( ionic bonding – entropy driven )
- Soft cations form more stable complexes with soft ligands ( covalent bonding – enthalpy driven )
- Hard-soft mismatches are not favored as not enough energy is released to overcome the high solvation energy of the hard species
The Chelate effect
- The stability of a complex increases with the degree of denticity of the complex
- There are three reasons that results in the extra stability
1. Probability
- Polydentate are less likely to be displaced as it requires the displacement of several donors before the polydentate is displaced
- This is a kinetic effect, so it will not affect the value of
2. Steric hinderance
- The bulky chelate hinders the attack of other ligands, thus prevents displacement from happening
- This is a kinetic effect, so it will not affect the value of
3. Entropy
- The substitution of a ligand of low denticity by one with a higher denticity is entropically favored
- The total number of molecules in the system through the substitution of a ligand of high degree of denticity. As a result, the entropy of the system increases
- This is a thermodynamic effect, so it will affect the value of