Matter can exist in different physical form even though they have the same chemical composition
- The physical form includes solid, liquid and gas
- It is also possible that one chemical substance have more than one solid form, and that each form is a different phase
A phase is a form of matter that has a uniform physical state and is distinctly separated from other phases
- Matter can exist in one or more phases simultaneously at equilibrium
¶ Gibbs Energy and Phase Stability
Not all phases are created equally, at a given condition, one phase can be made more stable than the others
- A substance will always adopt the phase that is the most stable under that particular condition
If the condition of the system changes, the phase that was originally the most stable may no longer be the most stable
- The substance will undergo a phase transition to attain the most stable phase
- A phase transition is a spontaneous process that converts the substance from one phase to another
Gibbs Free Energy is incredibly useful to understand how the phase changes depending on the pressure and temperature
- This is because the natural variable of Gibbs energy is also pressure and temperature
- The phase with the lowest Gibbs free energy will be the most stable
The Gibbs Free Energy is composed of the enthalpy and entropy term
- The entropy term will have a dominant effect at high temperatur
- The enthalpy term will have a dominant effect at low temperature
Solids have a large and negative enthalpy but a small entropy
- Solids are packed close together, so there is strong intermolecular interaction between particles, which contributes to a large magnitude in the enthalpy of formation
- The degrees of freedom of atoms and molecules within a solid is small as they are fixed in place. As a result, the entropy of solid is low
Gases have a small enthalpy but a large entropy
- Gas particles are far apart from each other, so the intermolecular forces between them is very weak, resulting in a small magnitude in the enthalpy of formation
- Gas particles have a high degree of freedom, so they have a lot of microstates, which explains the high entropy value of gas
Liquids have an intermediate enthalpy and entropy value
- The particles in a liquid are fairly close together, but they are able to rumble around, so they have a medium intermolecular interaction, which results in a medium magnitude in the enthalpy of formation
- The particles in liquid can move around but not as much as they can in a gaseous phase, so the entropy value is intermediate
¶ Temperature-Dependence of Gibbs Energy
A phase transition occurs at a characteristic temperature for a given pressure
- The characteristic temperature is the transition temperature
During phase transition, the temperature of the system remains constant, meaning that phase transition is an isothermal process
- Only when one phase has completely changed to another phase will the heat act to change the temperature of the system
- Phase transitions are inherently isothermal
A characteristic amount of heat is absorbed or released during a phase transition, we can define it as the enthalpy of transition,
- Enthalpies of phase transitions are formally defined for endothermic process, so they have a positive values
- For phase transitions that occur under the same conditions but in the opposite direction, the enthalpy of transition will be the negative of its endothermic counterpart
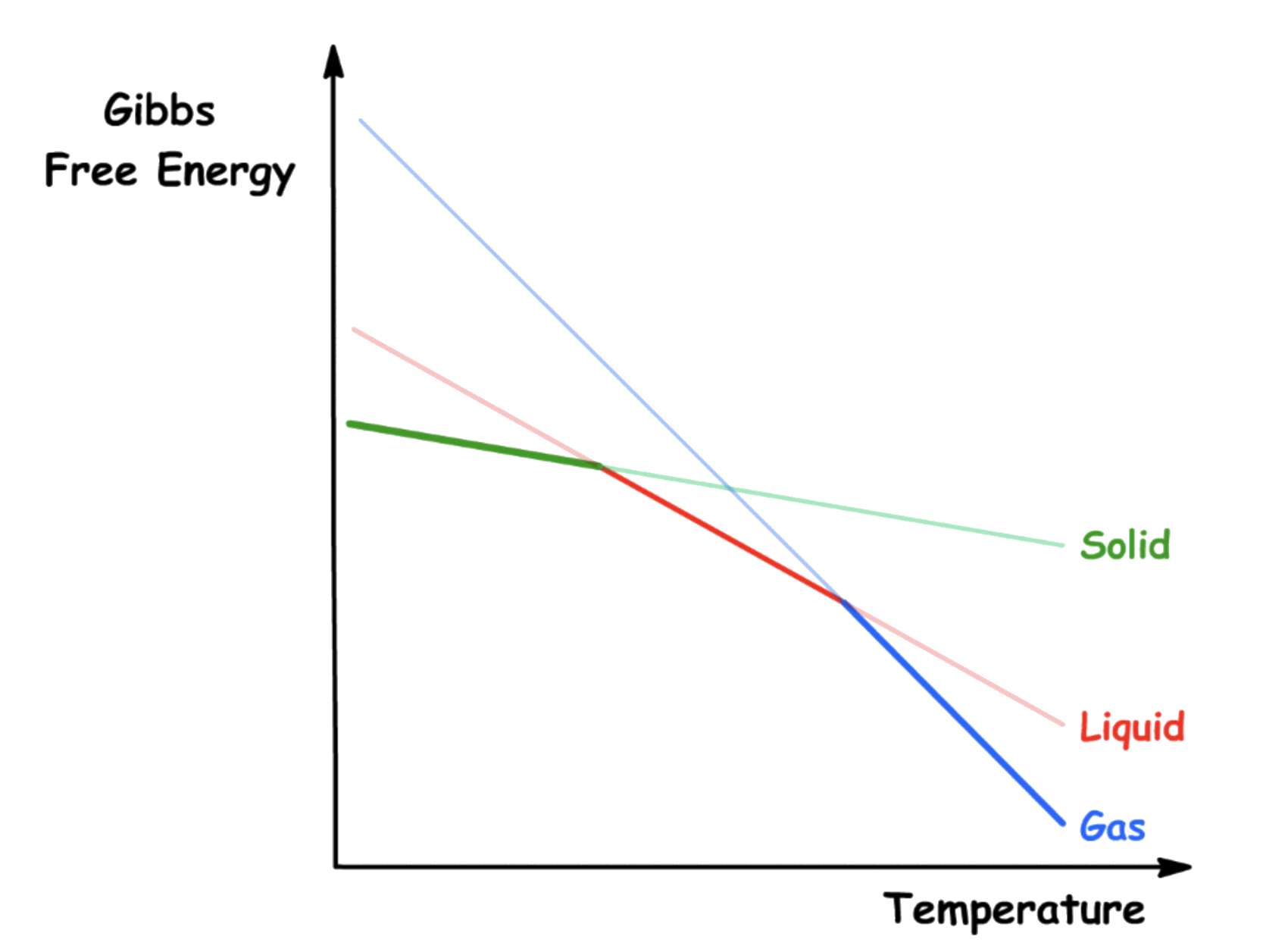
To understand how substances changes phase with temperature, we must understand how Gibbs energy changes with temperature
- From the Fundamental Thermodynamic Relations, we know what the rate of change of Gibbs energy with respect to the temperature at constant pressure is just negative entropy
- Since the entropy of all real substance is greater than 0, the Gibbs energy always decreases when the temperature is raised at constant pressure and composition
Note that the gradient of the function is related only to the entropy value of a particular state
- As established, the value of entropy increases when from solid to gas, so we should expect to see the slope to become steeper as we go from solid to gas
-- At , the effect of entropy is zero, so the value of the free energy depends entirely on the enthalpy of formation at absolute zero. This is consistent with the definition of Gibbs energy
- At the points where two lines intersect, the Gibbs energy of both phases at that stage is equal, so equilibrium is established
The phase that has the lowest Gibbs energy changes from solid to liquid to gas as we increase the temperature at constant pressure
- At low temperature, the effect of entropy is dominated by enthalpy. Hence, the magnitude of the free energy depends mainly on the enthalpy of formation. Since solids have the lowest enthalpy of formation, it is the most stable state at low temperature
- At medium temperature, the effect of entropy and enthalpy are equally important to the value of free energy. Since liquids have an intermediate enthalpy and entropy, it is the most stable state at medium temperature
- At high temperature, the effect of entropy is dominant. Since gases have the highest entropy, it is the most stable state at high temperature
¶ Pressure-Dependence of Phase
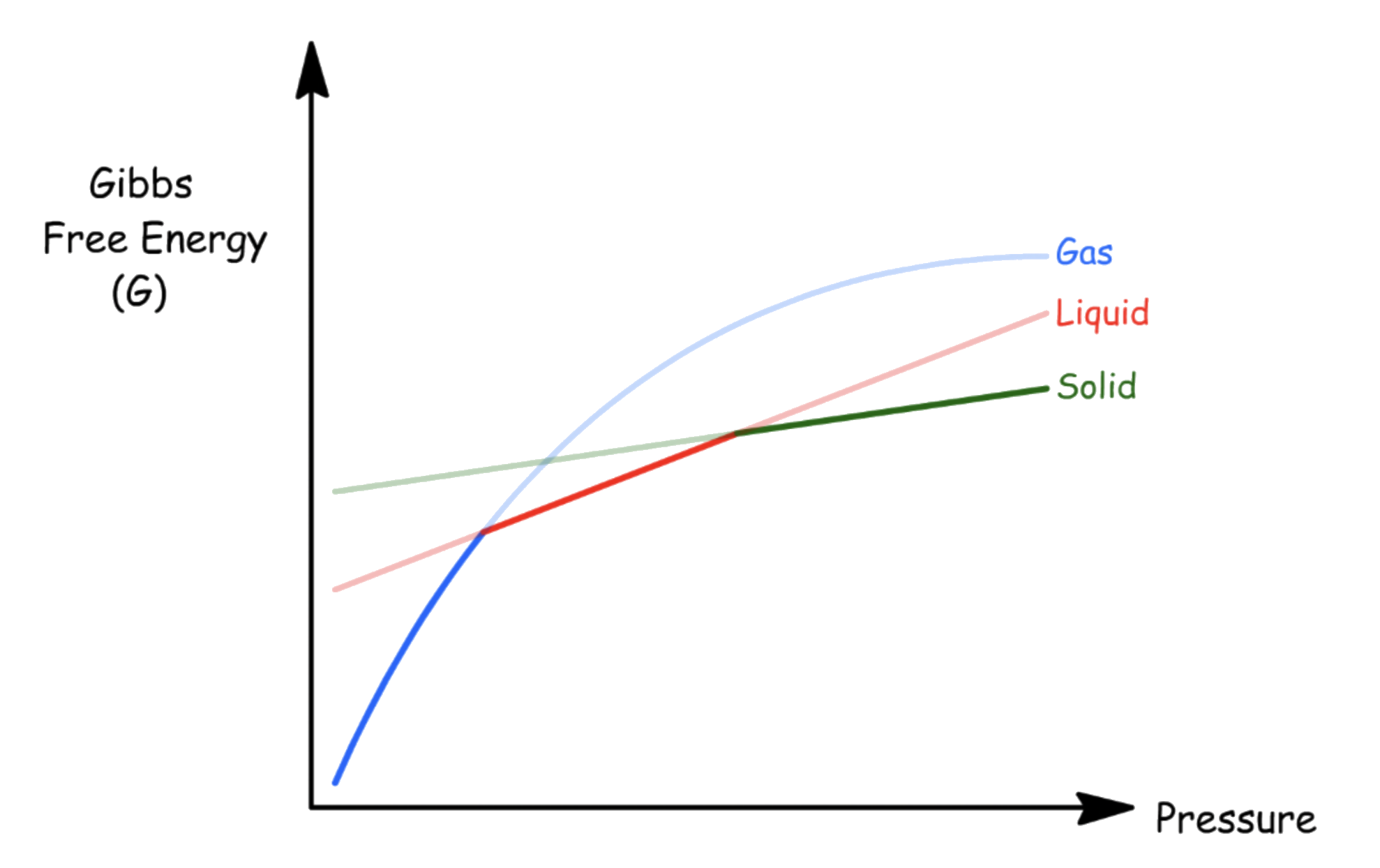
To understand how substances changes phase with pressure, we must understand how Gibbs energy changes with pressure
- From the Fundamental Thermodynamic Relations, we know what the rate of change of Gibbs energy with respect to the pressure at constant temperature is just volume
- Since the volume of all real substance is greater than 0, the Gibbs energy always increases when the pressure is raised at constant temperature and composition
- If we plot a graph of free energy against pressure at constant temperature, the slope of the graph will be the volume of that substance in a particular state in that particular pressure
-- At the points where two lines intersect, the Gibbs energy of both phases at that stage is equal, so equilibrium is established
Whether we can treat the volume as a constant depends on whether the volume of the substance in that state depends on the external pressure
- For condensed states, the volume is ( mostly ) constant as they are indifferent to the change in external pressure, so its graph is a straight line
- For gas, the volume is dependent on the external pressure, so the gradient of the graph decreases as the pressure increases
The phase that has the lowest Gibbs energy changes from gas to liquid to solid as we increase the external pressure at constant temperature
- Hence, things usually change from gas to liquid to solid as we increase the temperature
- An infamous exception is , this is because the volume of ice is greater than that of water, so an pressurizing ice will cause it to melt