¶ Reduction
Hydrogenation can reduce alkene or alkyne
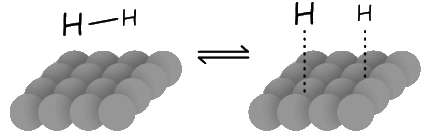
- Molecular hydrogen is adsorbed to the catalyst surface and dissociate into hydrogen
atoms
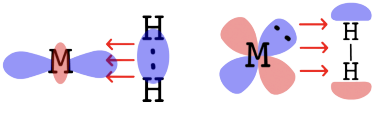
- The H - H bond is broken because electron density of the bonding orbital is donated to the metal. At the same time, electron density is donated to the orbital of dihydrogen
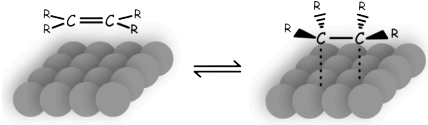
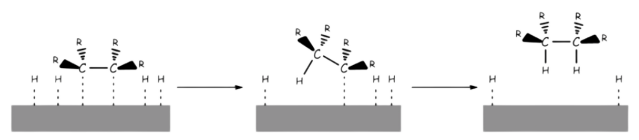
- The alkene or alkyne is adsorbed to the catalyst surface
- Hydrogen atoms are transferred from the metal to the carbons
- Reduction via hydrogenation therefore always add the hydrogens on the same face
- If we want to partially reduce alkyne to the an E-alkene, then we undergo a
hydrogenation with poisoned catalyst (Lindlar Catalyst; Pd(0) on a solid
support of CaCO3 deliberately poisoned with Pb(OAc)2.)
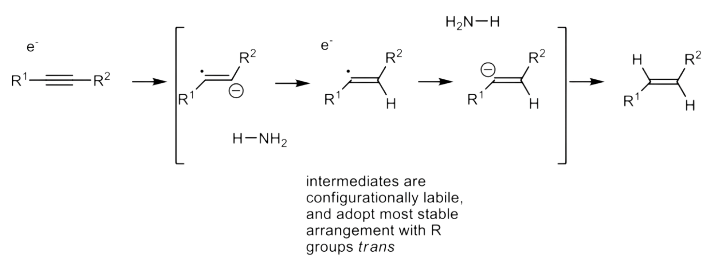
The Birch Reduction, also know as the Dissolving-Metal Reduction, can reduce alkene, alkyne
and even aromatics
Due to the absence of steric hinderance (e are too small), it will always form the
conformation that is the most thermodynamically stableWe can perform partial and selective reduction of arenes to 1,4-
cyclohexadienes using this reactionThe mechanism