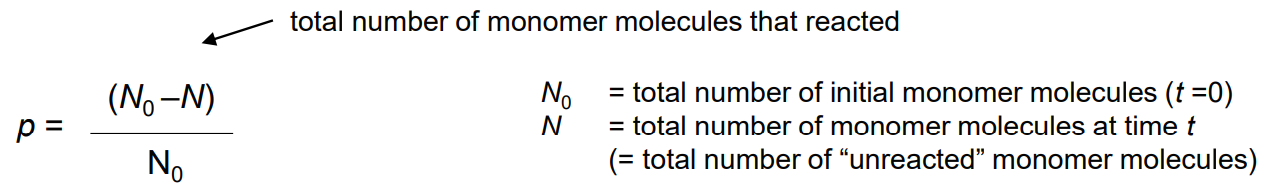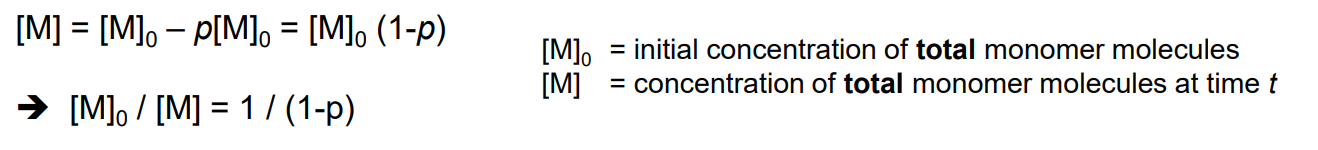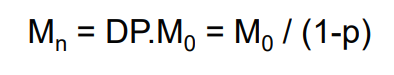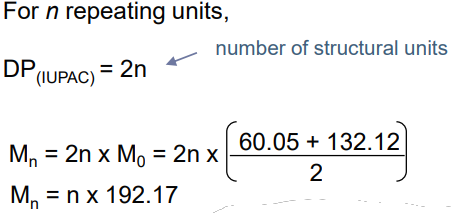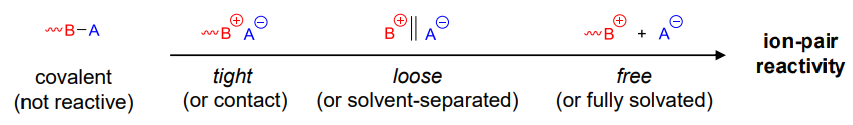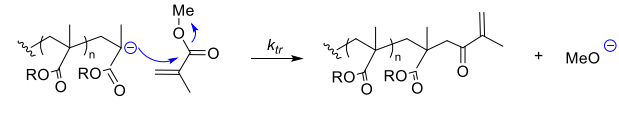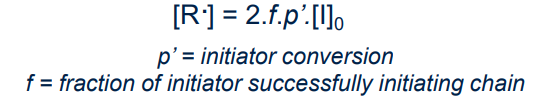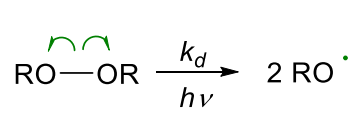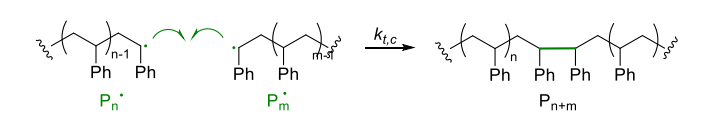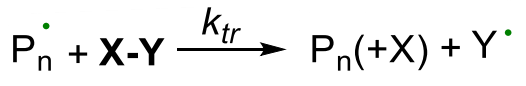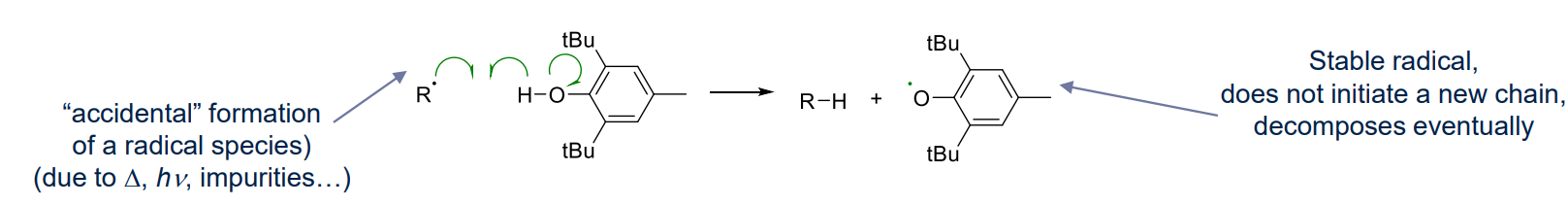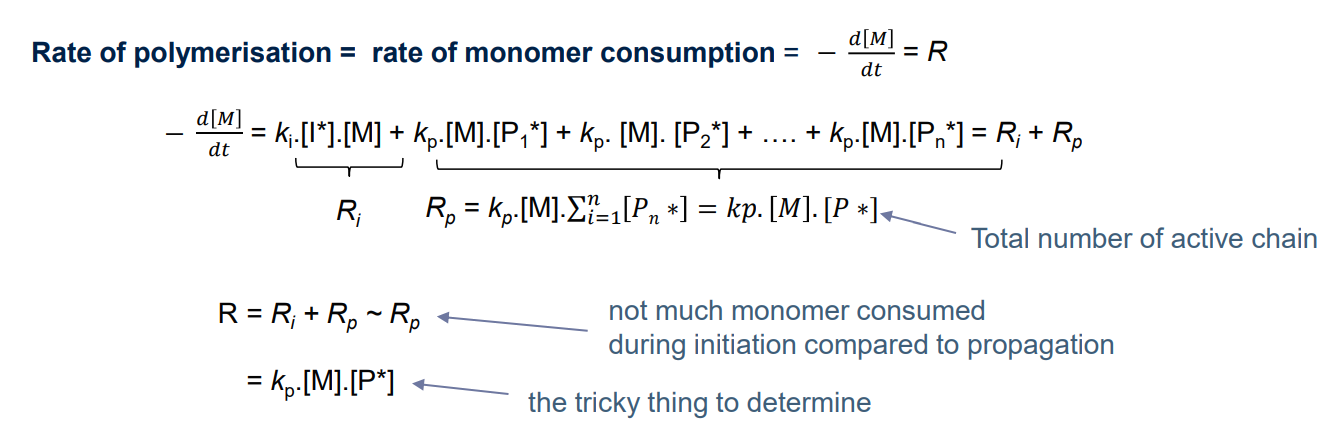¶ Fundamental
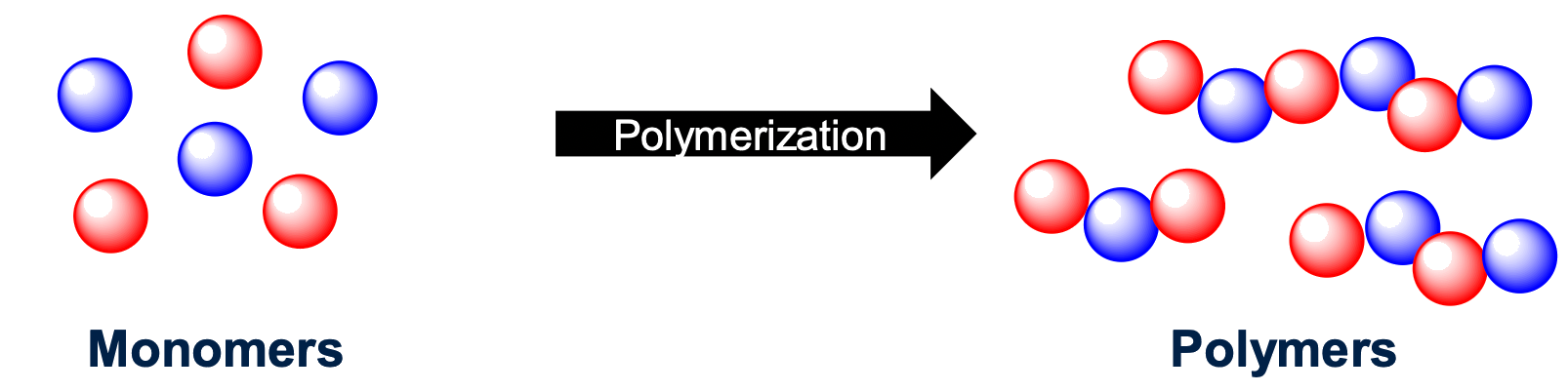
Polymers are a mix of macromolecules that are made of monomers
- Monomers are "building blocks" that can undergo polymerisation
- This can result in the formation of synthetic, semi synthetic & biopolymers
We investigate materials at 3 different levels and we will be diving deeper later on. Here is the general overview:
Monomer : reveals polymer composition
Polymer chain : reveals monomer arrangement: topology, regioselectivity & stereoregularity
Material : reveals chain arrangement: morphology, conformations
¶ Polymer composition
Different types of polymers
- Homopolymer - made of one type of monomer
- Copolymer - made of more than one type of monomer (examples on L1 slide 24)
- Terpolymer (copolymer) - made of three types of monomer
- Polymer blend - mixture of different polymers
¶ Polymer Chain Level
Regioregularity
Describes the bond between two monomers and the connection between two structural units ( for asymmetric monomers )
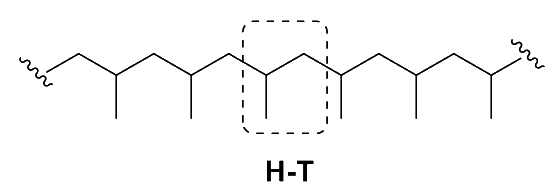
Head-to-Tail ( isoregic )
- In a head-to-tail polymer, the substituent of the monomers are located on alternate carbons in the polymer chain
- This results in a more extended polymer structure, with less opportunity for intra-chain hydrogen bonding
- This type of regiochemistry is often associated with lower melting points and lower crystallinity
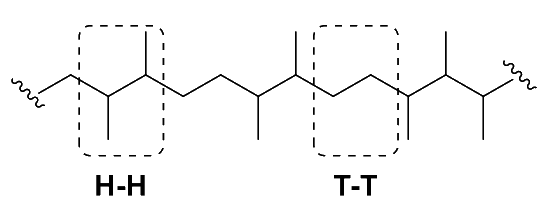
Head-to-head ( syndioregic )
- In a head-to-head polymer, the functional groups of the monomers are located on adjacent carbons in the polymer chain
- This results in a more compact polymer structure, as the functional groups can interact with each other and form intra-chain hydrogen bonds >- This type of regiochemistry is often associated with high melting points and high crystallinity.
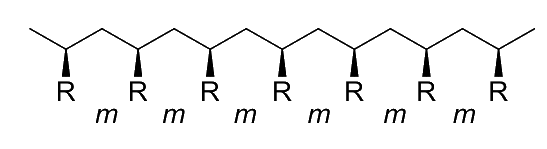
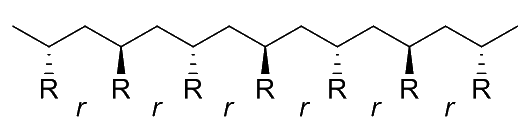
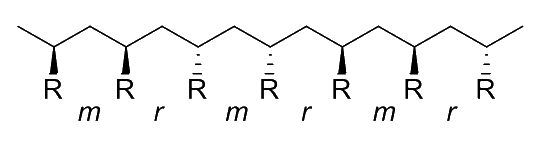
Stereoregularity ( Tacticity )
Stereoregularity, aka tacticity, refers to the spatial arrangement of the monomers in the polymer chain
- Specifically, stereoregularity describes the configuration pattern (order and disorder) of successive stereocentres
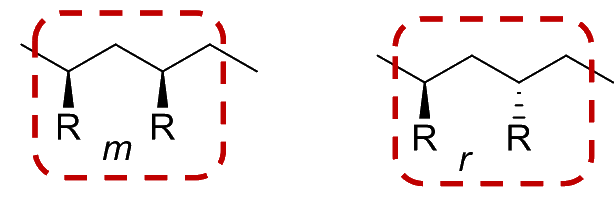
We can describe the relative orientation of any two consecutive R groups on the polymer as a diad
- A meso ( m ) diad refers to the arrangement where the two consecutive R groups are oriented in the same direction even if they are not on the same side of the polymer backbone
- A racemo ( r ) diad refers to the arrangement where the two consecutive R groups are oriented in the opposite directions even if they are not on the same side of the polymer backbone
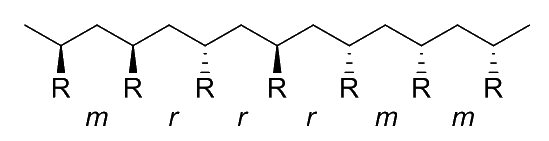
The collective arrangement of these diads gives us the tacticity of the polymer. There are four main types of tacticity:
Atatic
- The R groups are randomly arranged along the polymer chain, resulting in a disordered, amorphous structure
- This type of polymer has a random distribution of diads, which can include any combination of and
- This type of polymer has low crystallinity and is typically soft, with a low melting point
Isotactic
All of the substituent groups are located on the same side of the polymer chain, resulting in a regular, helical structure
- 100% diads
- This type of polymer often has high crystallinity and is stiff, with a high melting point
Syndiotactic
- The substituent groups alternate in a regular pattern along the polymer chain, resulting in a zigzag structure
- 100% diads
- This type of polymer also has high crystallinity and stiffness, with a high melting point
Heterotactic
- The substituent groups alternate in a regular pattern along the polymer chain, resulting in a zigzag structure
- 100% alternating diads ( )
¶ Material level
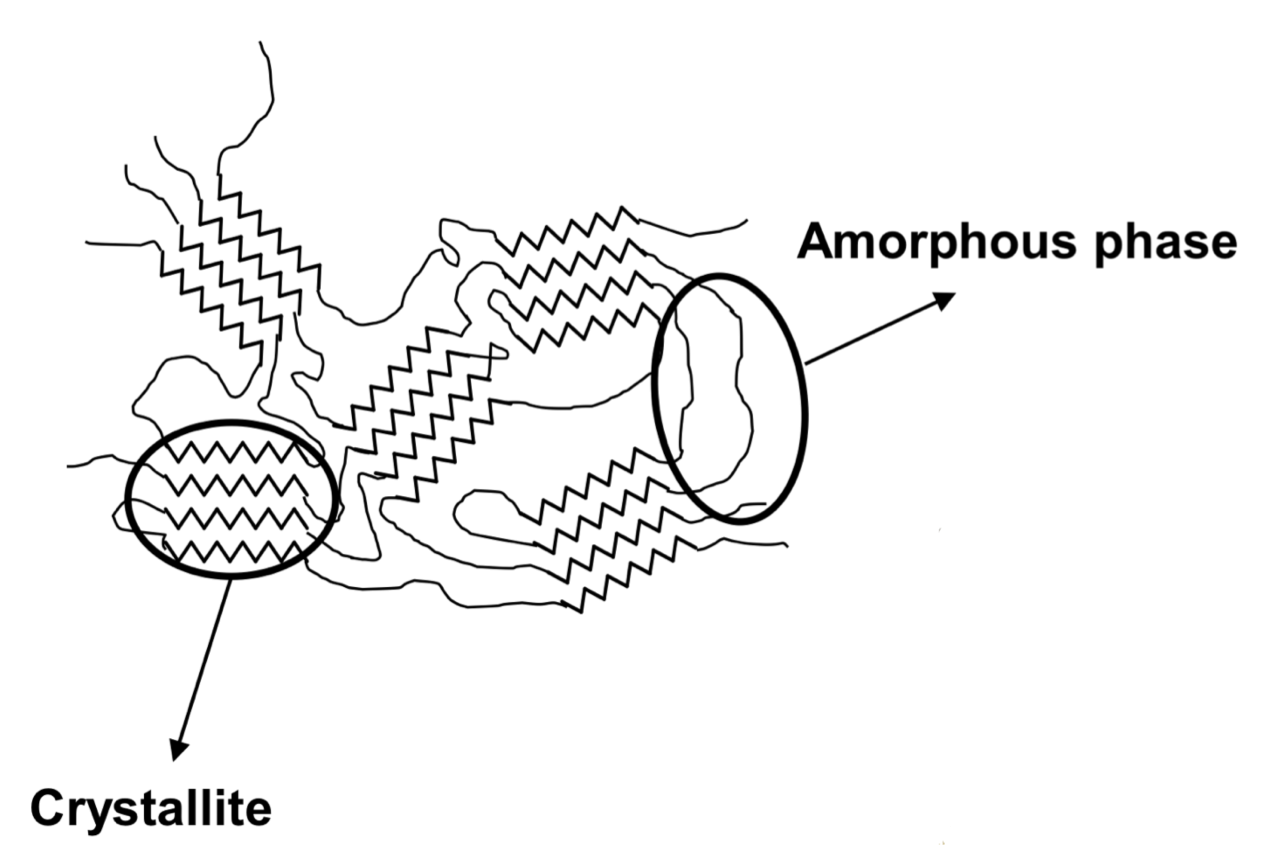
Crystallinity in Polymers
Due to the presence of extensive chain entanglements, polymers are seldom fully crystalline
- The degree of crystallinity is of considerable importance as it influences the properties of polymers
- There are two types of ordering present in polymers
- Crystallite domain - small sized ordered regions
- Amorphous domain - regions where chains can be incorporated in one/+ crystallites, contributes to some degree of mobility and its presence impairs the crystallinity of the material
In general, there are two types of polymers
- Amorphous polymers only contain the amorphous domain
- Semi-crystalline polymers contain both the crystallite and amorphous domain
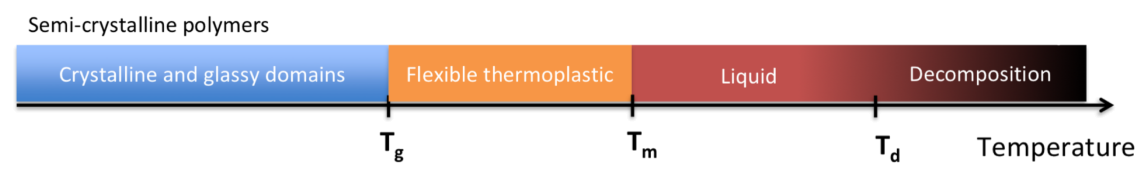
States of Polymers
Unlike small molecules which can only exists as solid, liquid or gas, polymers can exist in other three states :
Fluid
- Molecular behaviour: Random Brownian movement of chains
- The fluid goes from a "simple liquid" to an "oil" to "rubbery" as the molar mass of the polymer increases
Glass
- Molecular behaviour: No translational motion of the chains ; No long range segmental motion ( ≠ substituents )
- Brittle solid
- Often, but not always, has a transparent apperance
Semi-Crystalline
- Molecular behaviour: Ordered Regions
- Tough, hard, solid
- Often white 👴🏻
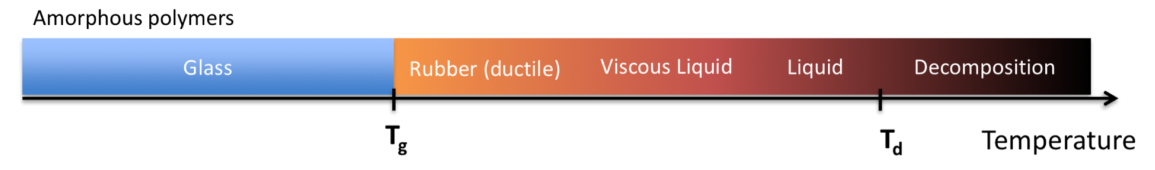
Thermal Transitions in Polymers
The term "transition" refers to a change of state induced by changing temperature
- Polymers undergo order transitions besides melting and each of these transitions has an associated temperature at which the process takes place
- Glass Transition Temperature
- The temperature associated with the change from a glassy state to a rubberlike state or vice versa
- For both amorphous and semi-crystalline polymers, marks the beginning of segmental chain mobility for the amorphous domain
- Crystalline Transition Temperature
- Obviously semi-crystalline polymers have a , while amorphous polymers do not
- Just like how marks the beginning of movements within the amorphous domain, is the temperature where the thermal energy overcomes the force within the crystallite domains and breaking up the crystallite domains
- Melt Transition Temperature
- Amorphous polymers liquify gradually over a broad temperature range above , so they do not have a
- Semi-crystalline polymers melt over a relatively narrow range, so they do have a
- Decomposition Temperature
- Both amophorous and semi-crystalline polymers have a
- This is the temperature where the thermal energy overcome the primary bonds holding the polymer together
- This is also why polymers do not exist in gaseous form, they break down before they can vaporize
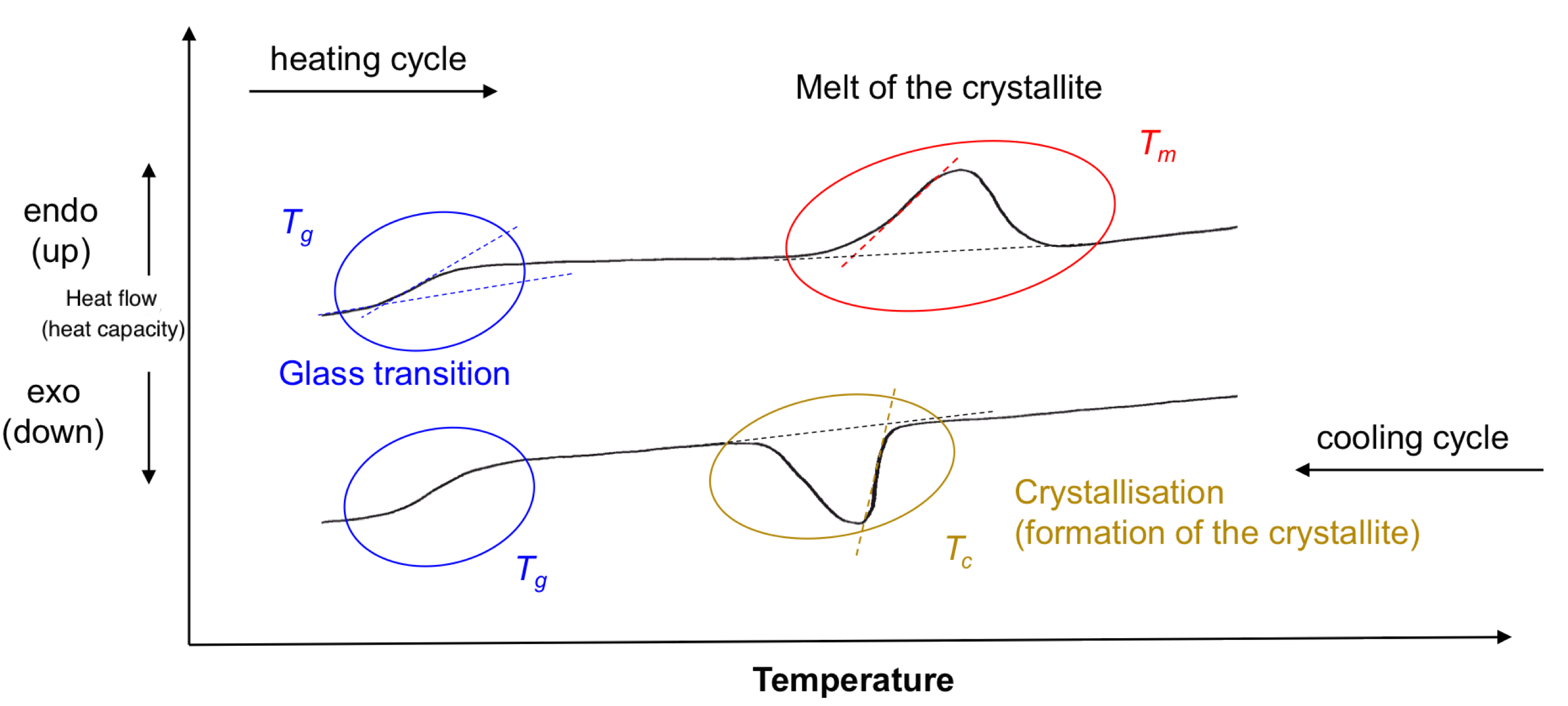
A DSC thermogram is a plot where the y-axis is the heat flow and the x-axis is the temperature
- During a DSC measurement, the difference in heat flow between a sample and a reference material is measured as a function of temperature. Any thermal events such as melting, crystallization, or chemical reactions in the sample will cause a change in heat flow, which is displayed as a peak or step in the thermogram
- The x-coordinate of each peak represents a transition temperature
- If the thermal event is endothermic, the peak will be a maximum. If the thermal event is exothermic, the peak will be a minimum
Factors influencing Transition Temperatures
Factors influencing Tg
marks the onset of molecular motion in the amorphous domain
- Therefore factors that affect rotation about links ( necessary for movement of polymer chain ) will also influence the
- In general, the is high when it requires a lot of energy for the chain in the amorphous region to move
Factors that influence :
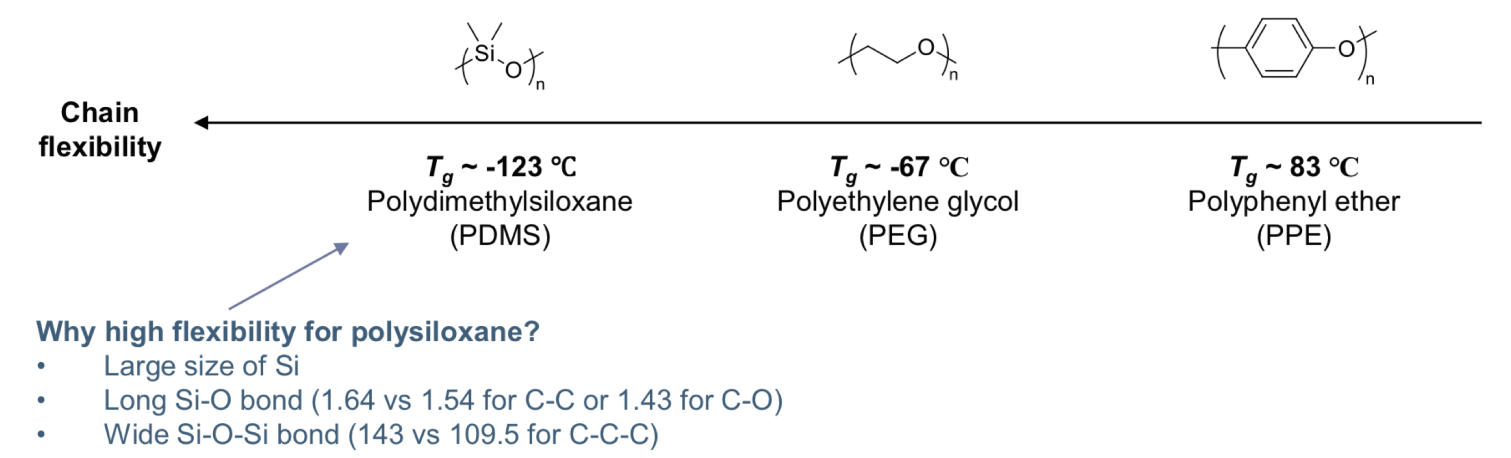
The more flexible the chain, the lower the . Chain flexibility depends on the rotational freedom about the skeletal bond :
The stronger the intermolecular interaction ( secondary interactions ) between the chains are, the higher the
Bulky side groups hinders rotation, which in turn raises the
Branching and crosslinking also hinders rotation, which in turn raises the
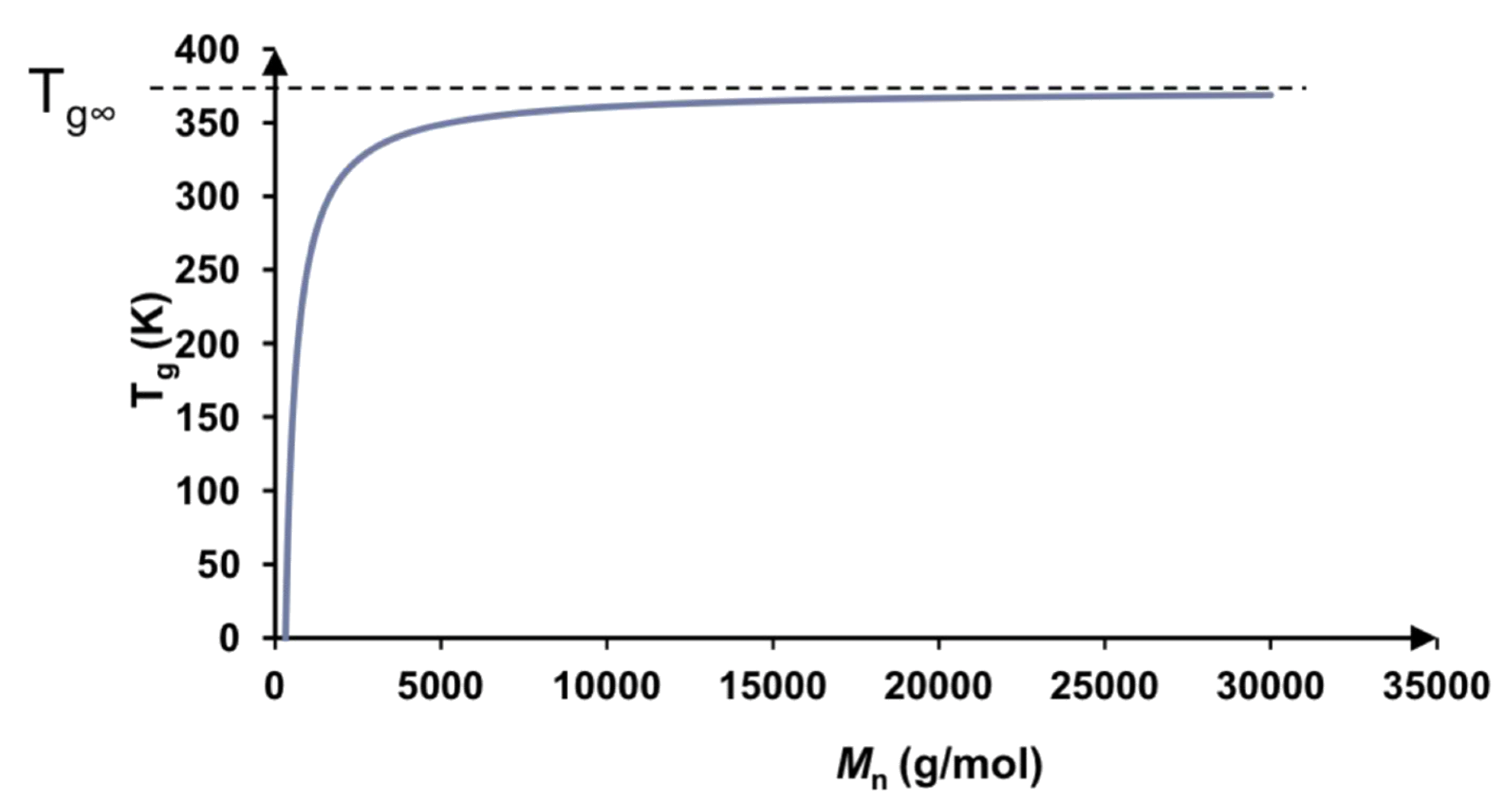
We know that the a high polymer molar mass implies a long chain length and therefore a high
- We can quantify the relation between molecular weight and using the Flory-Fox equation
Factors influencing Tm
Factors affecting can be derived from thermodynamics
- At , solid and liquid state is at equilibrium and therefore the free energy change must be zero:
- The melting temperature is therefore related to the change in enthalpy and entropy associated with melting:
- Tm is therefore high when the chain ordering is low ( small ) and the secondary interactions is strong ( large )
The factors influencing Tg also influences Tm, but in different proportions
- low low ; high high
Factors influencing Tc
The factors that influence include both thermodynamic and kinetic aspects
Thermodynamic aspects:
- Generally, polymers with higher molecular weight, more branching, or more rigid chemical structures have higher values
- Strong interchain interactions lead to higher values
- Higher degrees of crystallinity result in higher values.
- Note that high Tm ≠ high Tc
Kinetic aspects:
- The easier or faster it is to crystallise a polymer, the higher the ( more flexible chain increases crystallisation tendency )
¶ Polymer Molar Mass
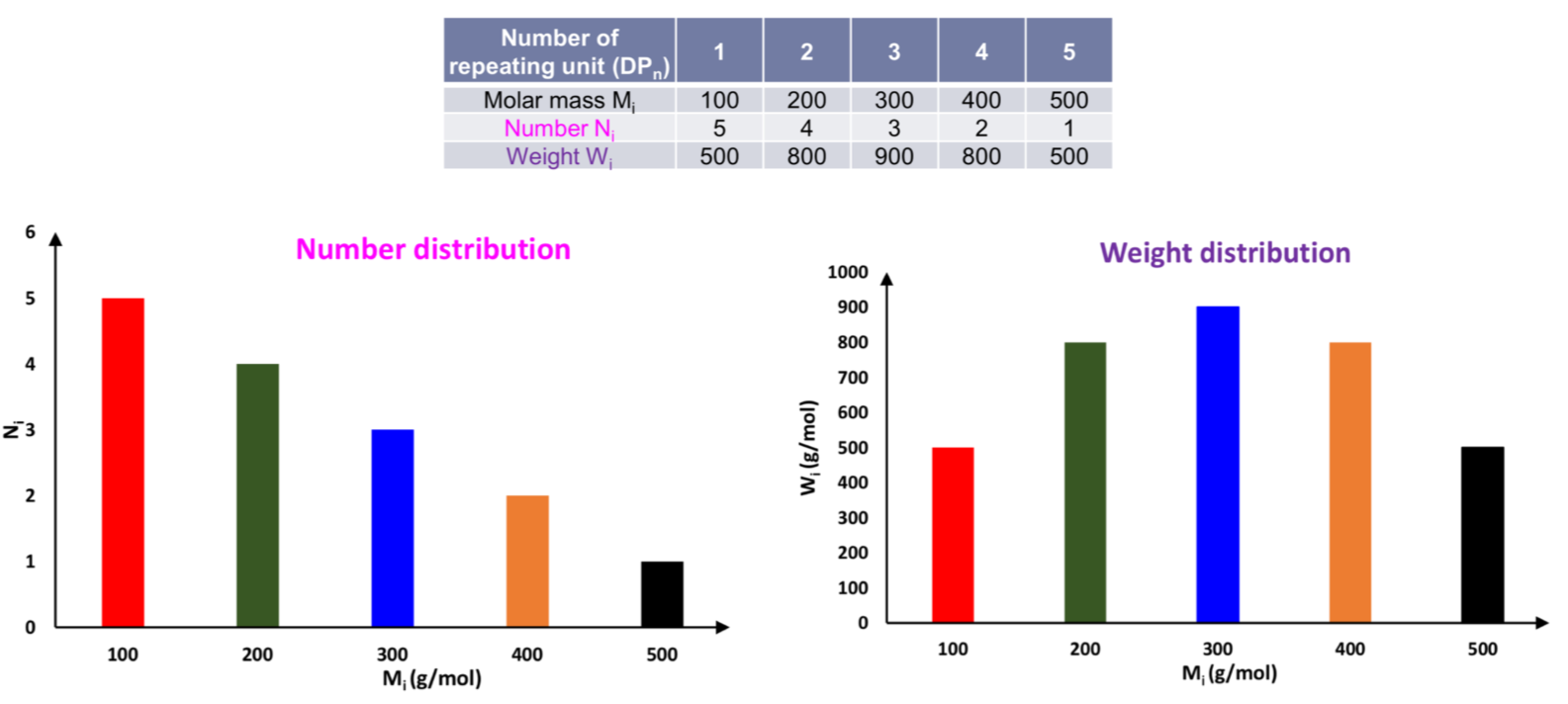
Not only are polymer molar mass very large, the molar mass within any polymer sample is not uniform
- Owing to this heterogenity, the value assigned to the molecular weight of a polymer depends on the way in which the heterogenity is averaged
¶ Number-Average and Weight-Average Molar Masses
Arithmetic Means
The distribution of molecular weights in a polymer sample is commonly expressed as the proportions of the sample with particular molecular weights
- The various molecular weight averages used for polymers can be shown to be simply molecular weight distributions
Suppose a polymer sample contains a total of units of molecules, where units are associated with molecules with molar mass of , units are associated with molecules with molar mass of , and so on
- The arithmetic mean molar mass is then the total measured quantity divided by the total quantity of molecules
- The ratio represents the fraction of units contributed by the chain with molar mass . Let us denote this fraction as
- Note that we didn't specify what the unit of measurement is ( it can be volume, mass, number etc. )
- Depending on what unit we are using, the fraction will take on different meaning ( volume fraction, mass fraction, mole fraction etc. )
- Substituting different into the equation will give us different Average Molar Masses ( Volume-Average Molar Mass, Weight-Avergae Molar Mass, Number-Average Molar Mass etc. )
Number-Average Molar Mass
The Number-Average Molar Mass is obtained by substituting in mole fraction in 's stead
- The mole fraction of species i is defined as the mole number of species i divided by the total mole number of the polymer sample
- We can therefore also express the Number-Average Molar Mass as such
- The product of and is just the total weight of species , : ()
Weight-Average Molar Mass
The Weight-Average Molar Mass is obtained by substituting in weight fraction in 's stead
- The weight fraction of species i is defined as the total weight of species i divided by the total mole weight of the polymer sample
- We can therefore also express the Weight-Average Molar Mass as such
- The total weight of species , , is just the product of and is just the ()
Brief Summary
¶ Average Degree of Polymerization
The fact that the molar mass of a polymer sample is not homogeneous implies that the degree of polymerization is also not uniform
- We therefore define the Average Degree of Polymerization, \text
- Just like there are two types of average molar mass, there are two types of average degree of polymerization, namely the number-average and weight-average degree of polymerization, and
- We usually use the number-average degree of polymerization , where represents the average number of monomers in a chain
¶ Molar Mass Distribution
The distribution of molar mass is different depending on the parameter we are choosing
The ratio of to is the dispersity
- Dispersity is a measure of heterogenity which quantifies the molar mass distribution
- Can be ploted to show how the number of molecules varies with molar masses
- Mn is the peak and Mw is slightly on the right
- Đ = 1 → monodisperse polymers = well controlled polymerisation
¶ Reactions and mechanisms
There are two primary methods for polymer chains formation
- step growth polymerisation
- chain growth polymerisation
¶ Step growth polymerisation
- via formation of dimers, trimers, tetramers, etc….
- via reaction that are identical in rate and growth reaction mechanism
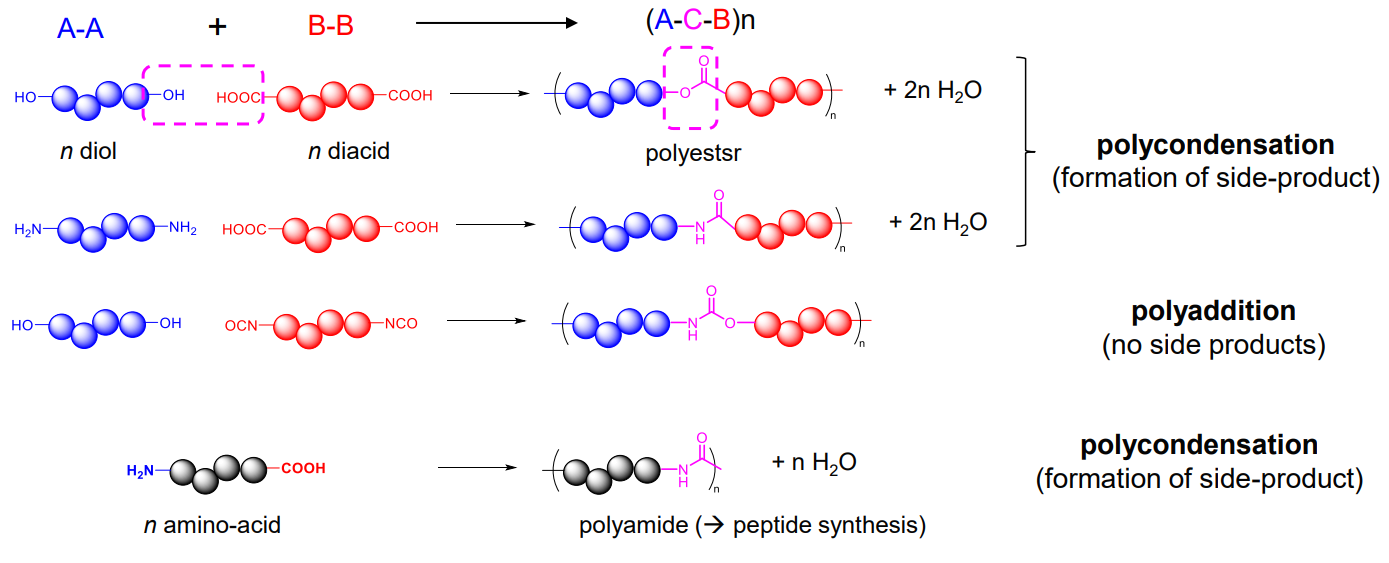
In general, there are two types of monomers when it comes to step-growth polymerization
- AA / BB types
- AB types
General reactions
Common reactions:
- Polycondensation
- number of monomers ≠ atoms in repeating unit
- elimination of H2O as small molecule side product
- pros: under Le Chatelier’s principle, an eqm recation can achieve increased polymer molar mass
- cons: energy demanding, waste
- Polyaddition
- no small molecule side products
- number of monomers = atoms in repeating unit
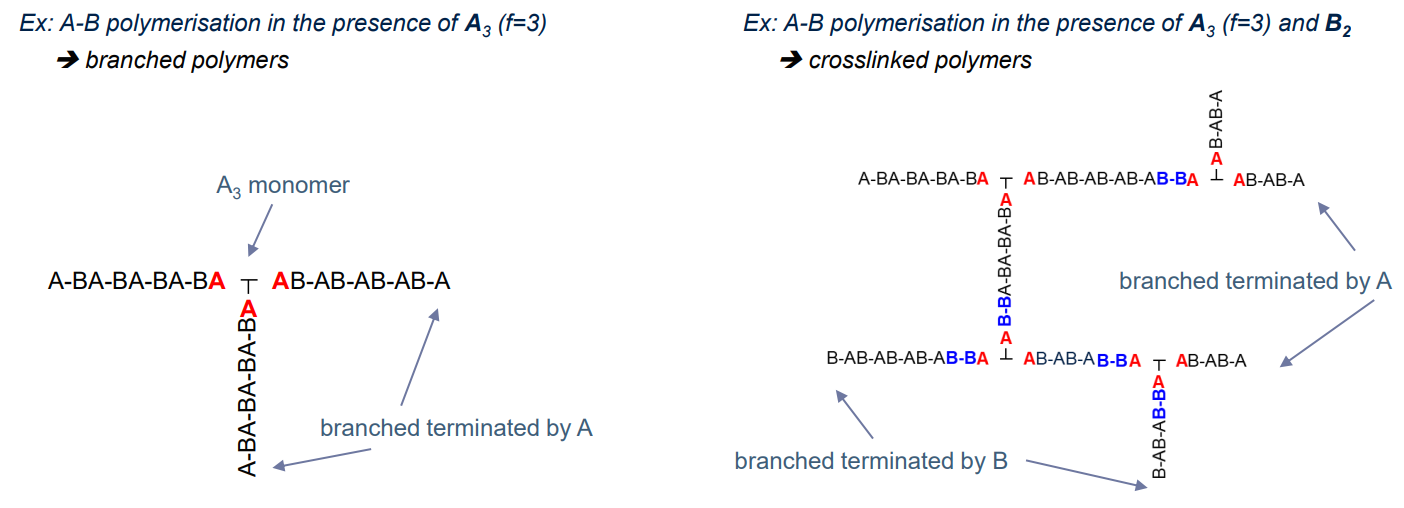
Non-Linear Polymers
Branched and crosslinked polymers are non-linear, from monomers with more than two functional groups (monomer functionality > 2)
¶ Calculations
Extent of reaction ( p )
also known as monomer conversion
Value of p is useful to find concentrations of monomers
A high conversion (extent or reaction p) is needed to reach high molar masses
Degree of polymerisation (DP)
- the average number of structural units per polymer chain
- DP can be different from the number of repeating units (n) e.g. copolymers
Value of p and DP is useful to find molar masses
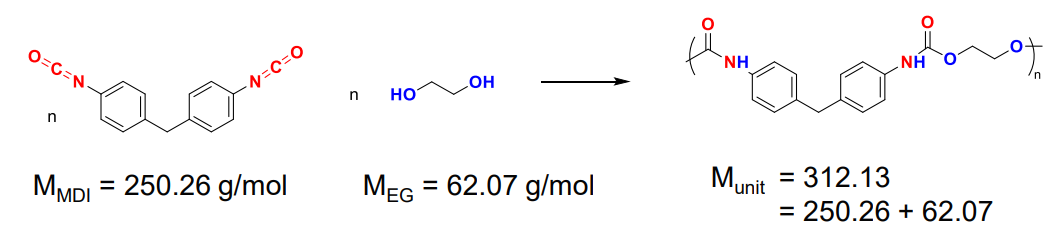
Example with PET
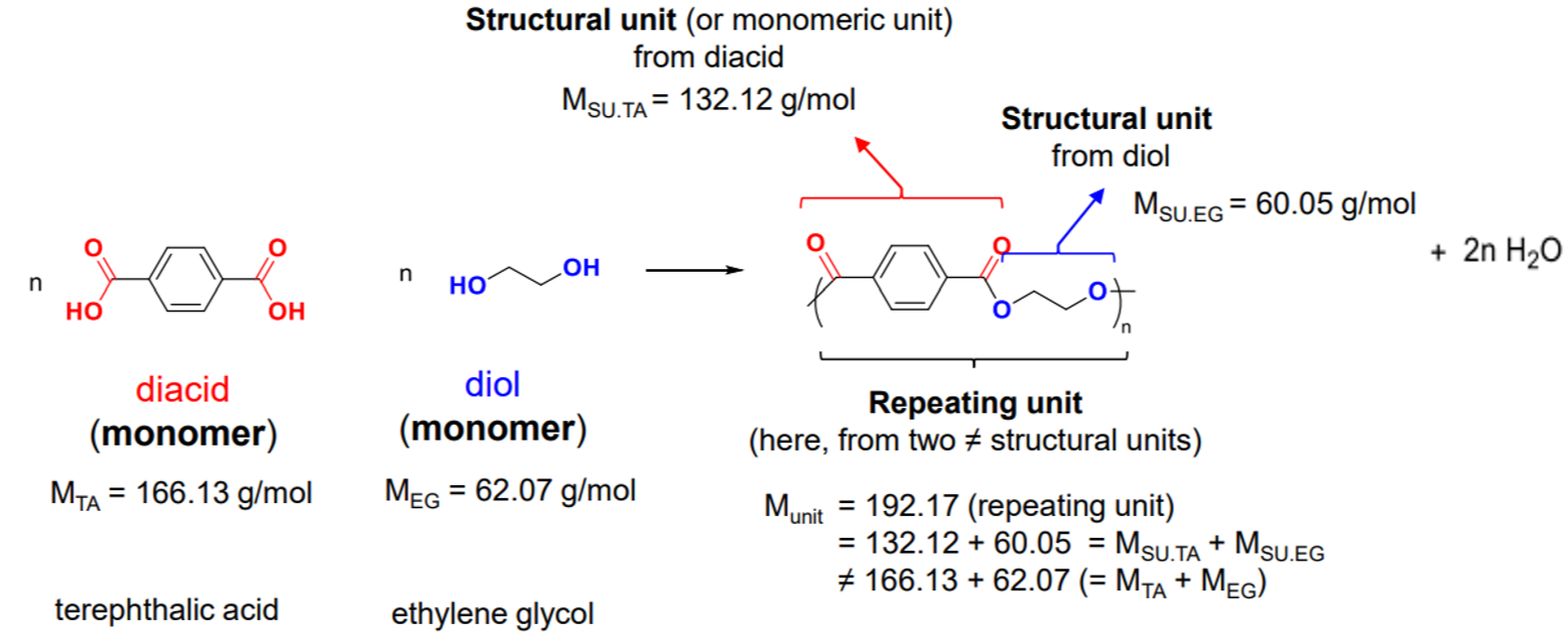
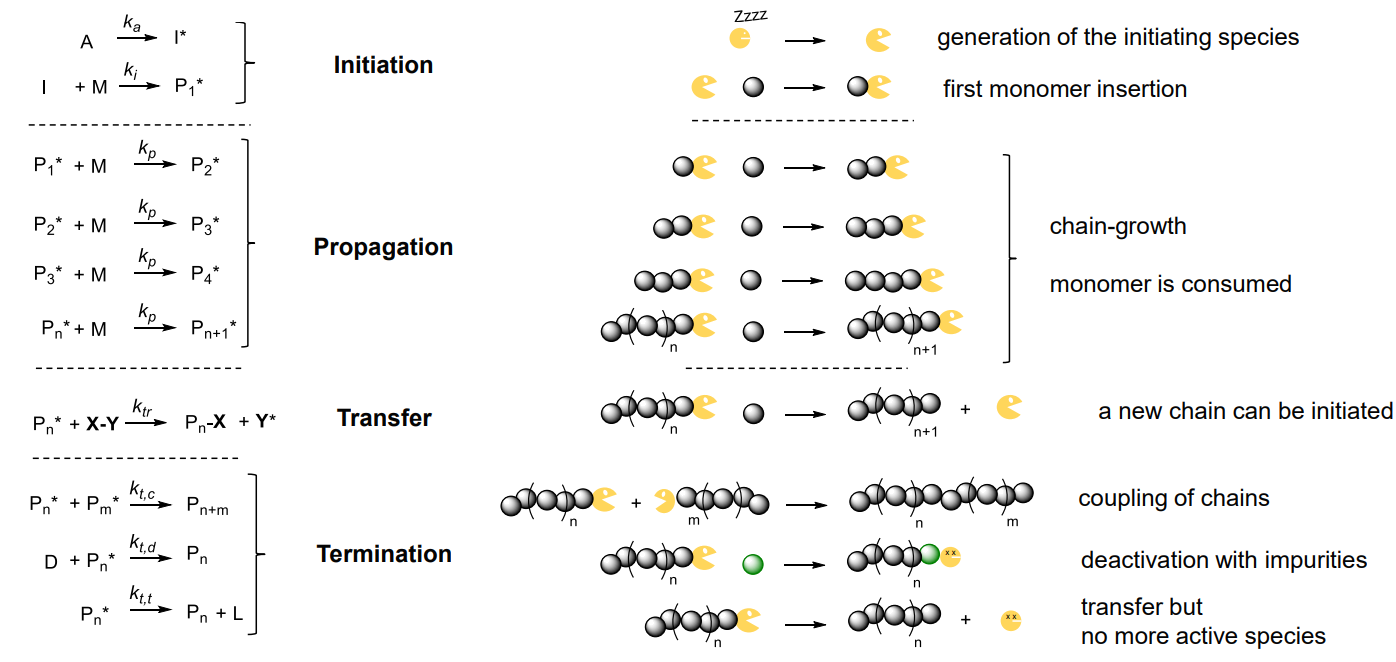
¶ Chain growth polymerisation
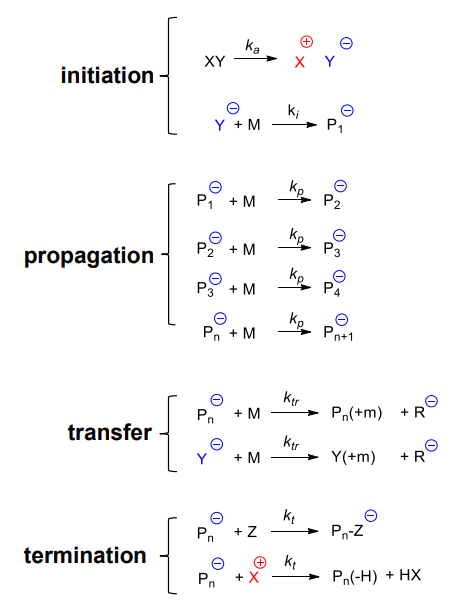
Polymer chain growth proceeds between monomer(s) and reactive site(s) on the polymer chain, with polymer chain regeneration of recative sites at the end of each growth step
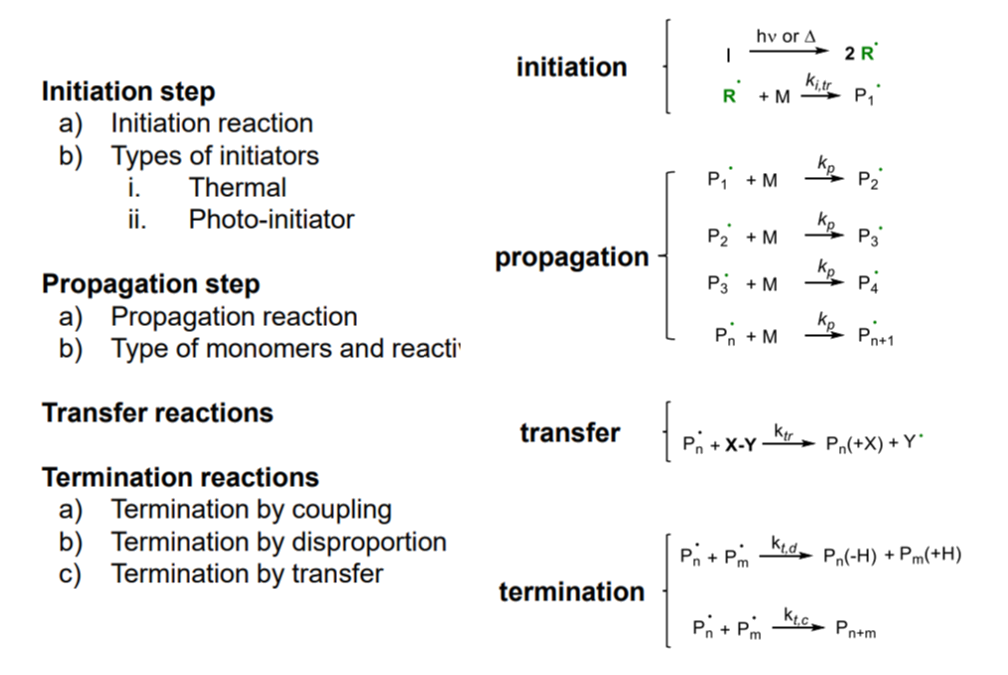
Reaction steps:
- initiation: I* (initiating species) generation & M (monomer) insertion
- propagation: another M insertion to Pn* (active chain / propagating species)
Chain breaking steps:
- transfer: active chain transferred to another species intiating new chains
- termination: ends chain by reactive chain deactivation
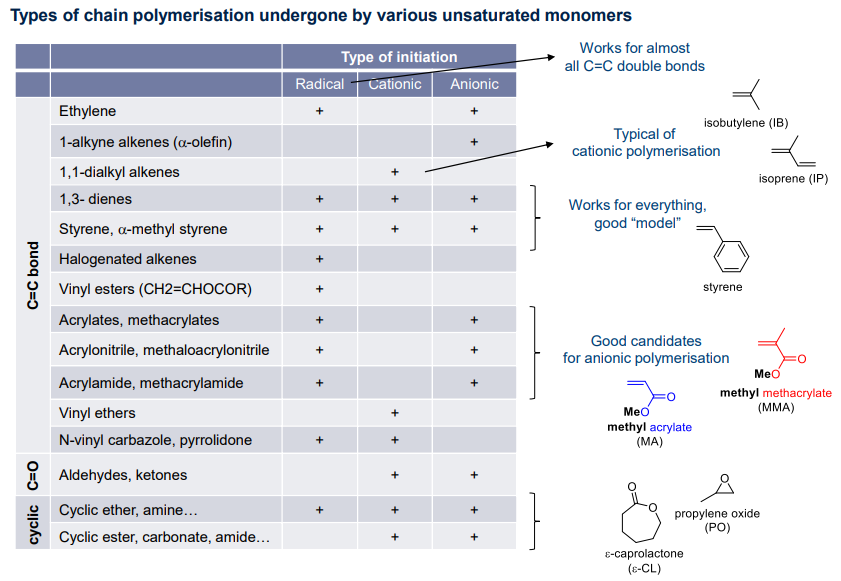
Types of Chain Growth
Different chain growth polymerisation reactions are determined by the types of and . Resulting in different routes: radical, cationic, anionic
Ionic Chain Polymerisations
Chain Polymerisation can either be cationic or anionic in nature
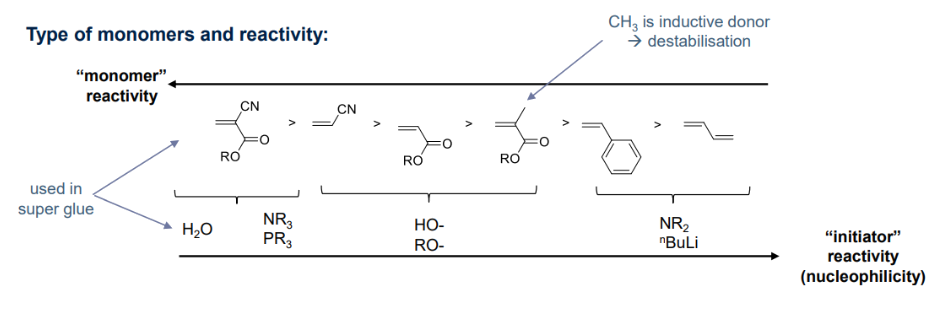
- Monomer substituent:
- EDG → cationic
- EWG → anionic
- Active chain stabilisation considering mesomeric & inductive effects:
- charge delocalisation, resonances of propagating specieices to 'activate' the monomer
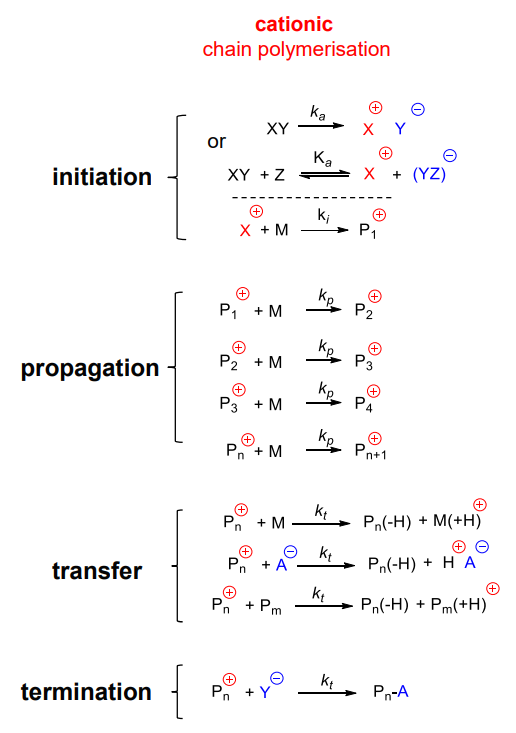
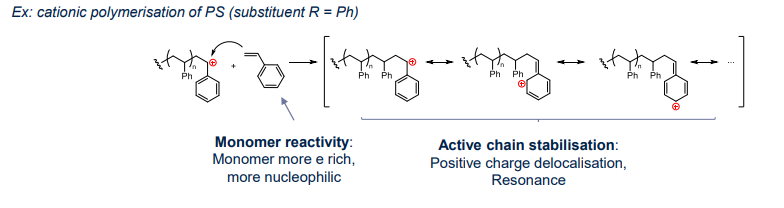
Cationic Chain Polymerisations
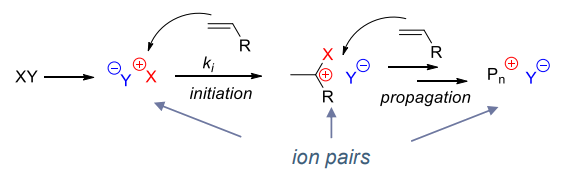
¶ Initiation step
Formation of ion pair to react with the monomer
The bonding of the ion-pair affects reactivity
It is important to use anion that doesn't inhibit or affect reactivity, this can be controlled with solvents
- polar solvents shifts reactivity to "free ion pair"
- prefer non nucleophilic/protic solvents e.g. avoid alcohol, amine, ketone, as they would react with the cationic chain end
Good initators
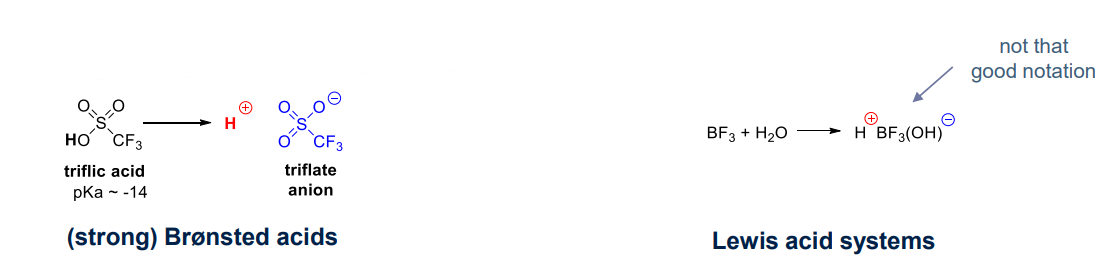
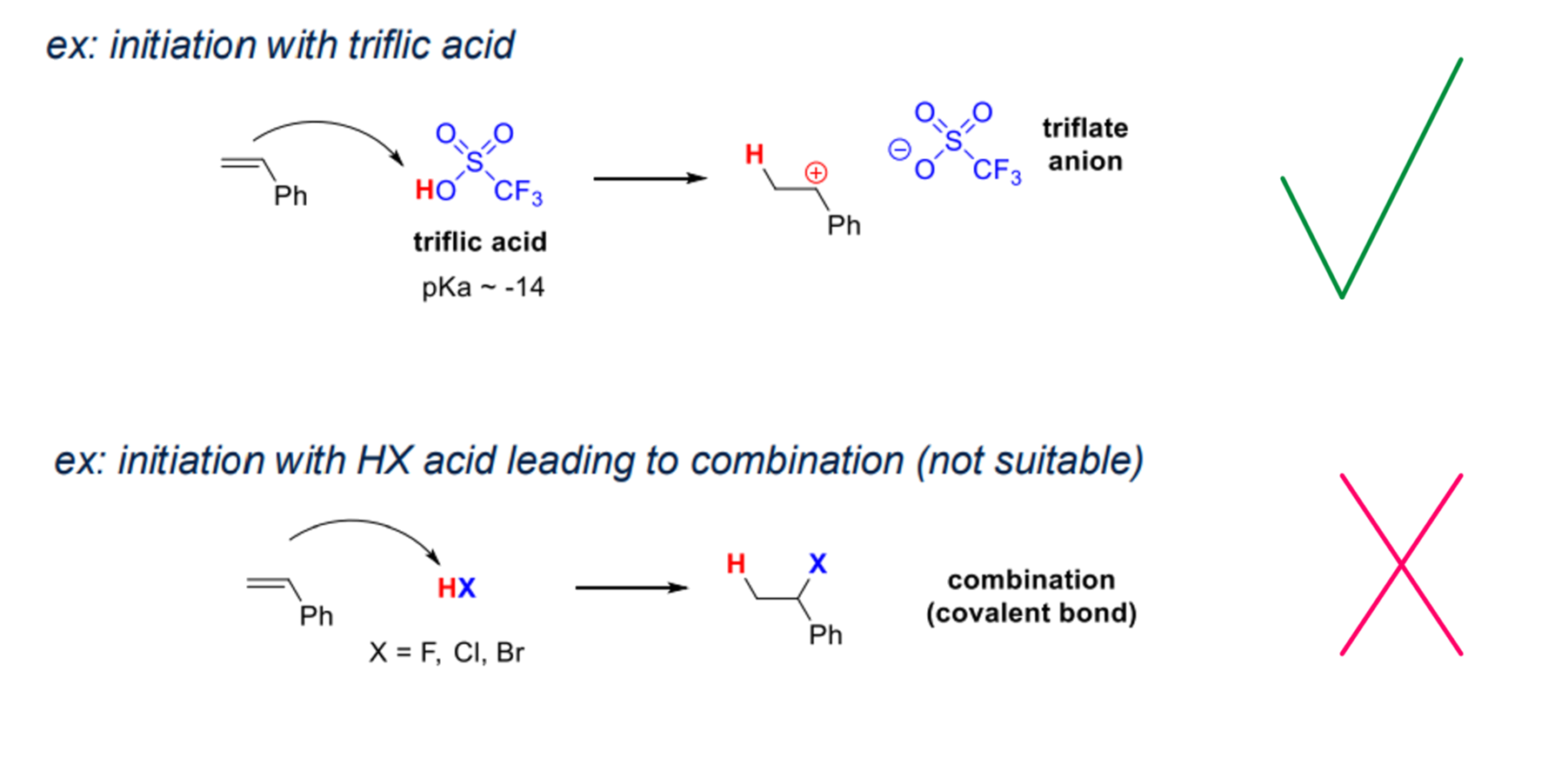
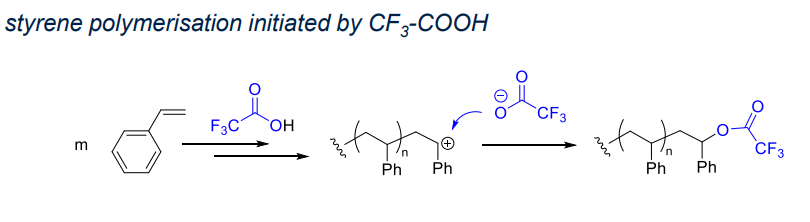
- Brønsted acid:
- a non nucleophilic counter anion
- e.g. HX will add to the double bond rather than initiate
Examples:
- triflate anion is soft with negative chrage delocalised on anion so it is a poor nucleophile
- covalent bond kills polymerisation (no initiation)
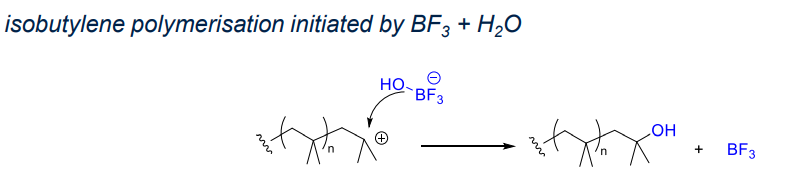
- Lewis acid system:
- low temperature, leading to high yeild polymers of high molecular weight
- reaction between initiator and co-initator
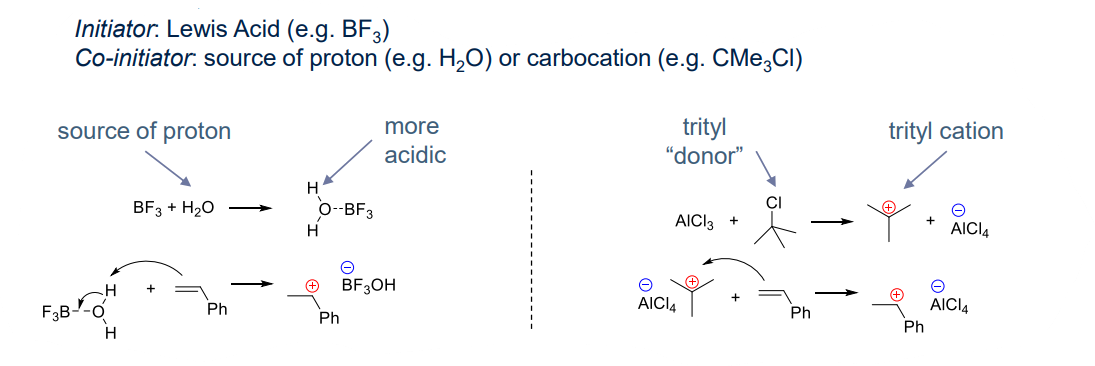
¶ Propagation step
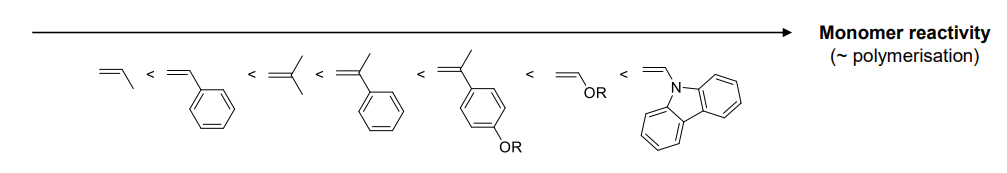
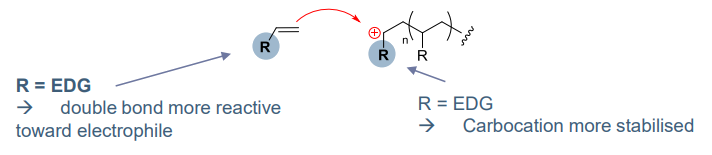
Reactivity:
The Reactivity of the propgation step depends on carbocation stabilisation by the substituent
- EDG mesomeric & inductive effects
- increase nucleophilicity of monomer
- stabilising carbocation
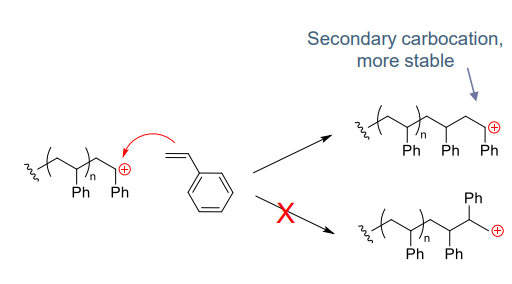
- Regioselectivity formation of most stable (most subtituted) carbocation
- Rearragnement reaction can occur (enamine)
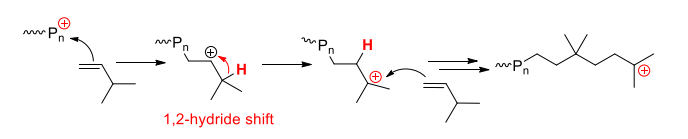
Rearrangement (isomerisation polymerisation)
When intramolecular rearrangement ( isomerism ) occurs, driven by the formation of more stable carbocation and/or ring strain release via
1,2-hydride (H-) shifts (before propagating in the example below)
1,2-methide (CH3-) shifts
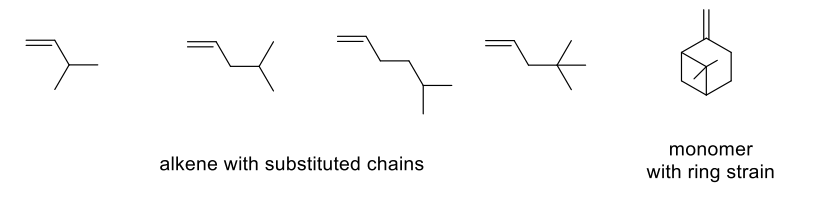
Monomers which undergo rearrangment:
¶ Transfer step
Occurs to monomers, anion, polymer in different ways
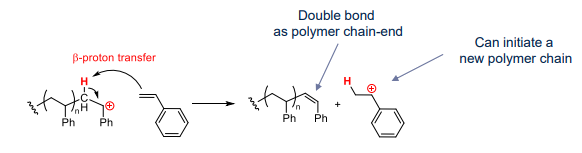
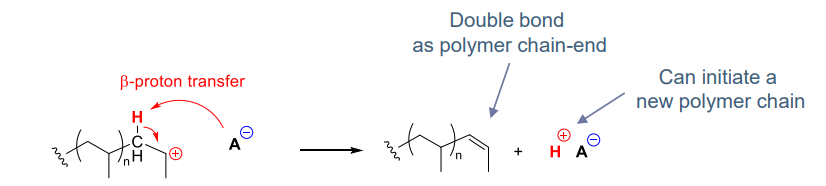
what happens when transfer step is to monomer:
- beta proton transfer generates new chains which limits polymer molar mass
- polymers formed can have different chain ends (e.g. a-methyl-styrene)
- beta proton transfer is difficult to avoid, so we work at low temperature to prevent transfer
- difficult for living polymerisation to occur
what happens when transfer step is to anion:
- beta proton transfer is difficult to avoid, so we use soft and weakly basic anion to reduce transfer
- difficult for living polymerisation to occur
- hard acid can potentially reinitiate chain (referred to as simple termination)
what happens when transfer step is to polymers:
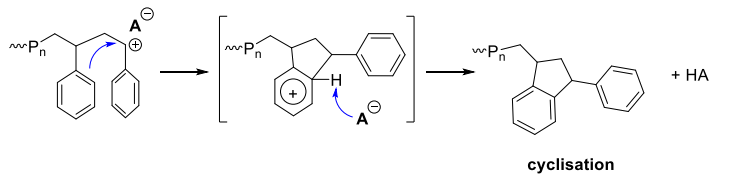
via intramolecular electrophilic aromatic substitution SEAr leading to cyclisation
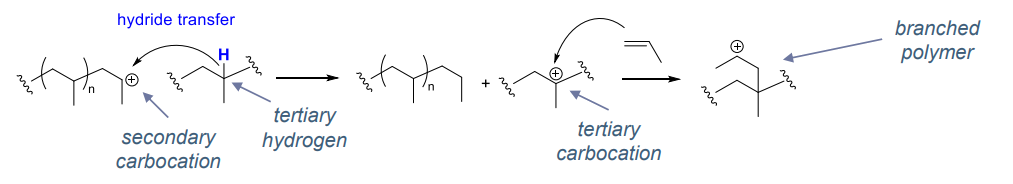
intermolecular hydride transfer to polymer leading to branching
- common with poorly stabilised carbocations (e.g. 1-alkene)
- Via other mechanisms, e.g intermolecular SEAr
¶ Termination step
Forms covalent bond with the aninon resulting in zero active centres
Examples:
- generating Lewis acid, which doesn't not initiate any polymerisation
Anionic Chain Polymerisations
¶ Initiation step
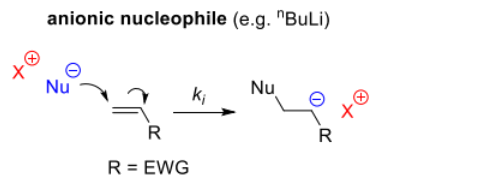
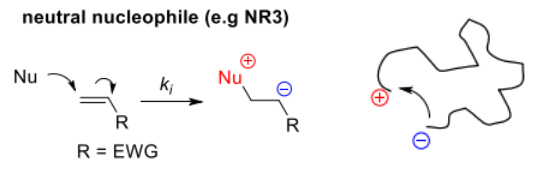
As classified previously, R group of the monomer is stabilised by EWG (cyano, carboxyl, vinyl or phenyl groups) when reacted with:
- anioinc nucleophile (most common initaitor)
- neutral nucleophile, highlights the importance of solvent
- generates zwitterions ∴ the polymer can cyclise
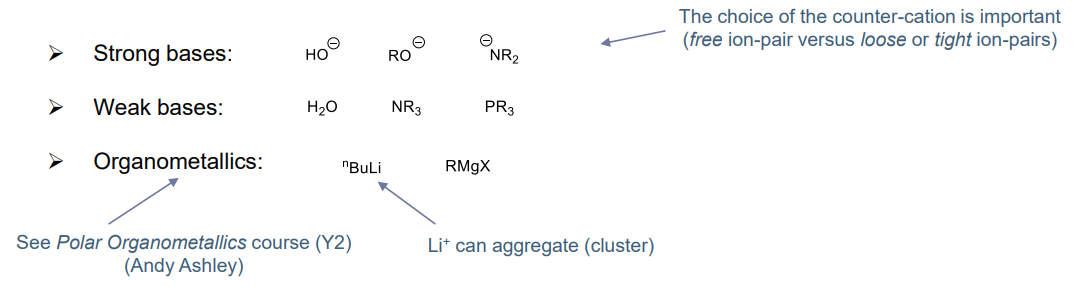
Initiator types
- basic and/or nucleophilic species (mainly)
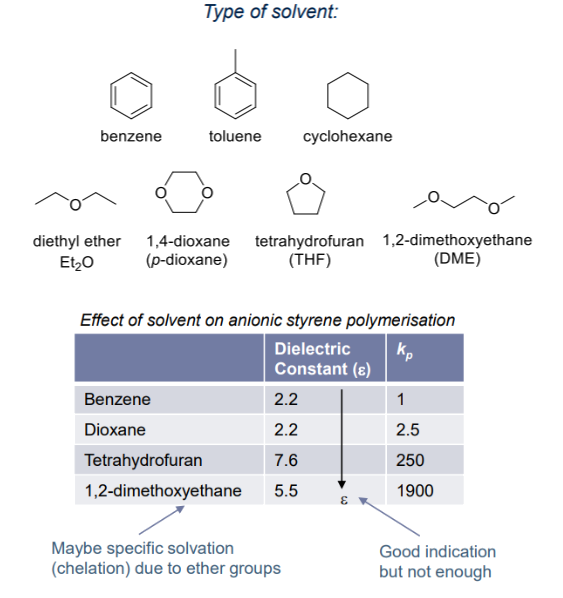
Effects of solvents
When a solvent dissolves ionic species, it is known as solvation. High polarity solvent is wanted for ion-pair dissociation, which allows for stability towards reactive carbanion.
- The effects of solvent is more pronounced than cationic reaction where counter-anions are usually soft
- Encourages anioinc polymerisation with greater range of counter-cations: from hard to soft (Li+ to Cs+)
- Increases reaction rate: free ion-pair >> loose ion-pair > tight ion-pair
Types of solvents:
- Non-reactive towards carbanion → non-electrophilic (e.g. not CH2Cl2 ) and non-protic (e.g. not ROH)
- Aliphatic and aromatic hydrocarbon
- Ethers
¶ Popagation step
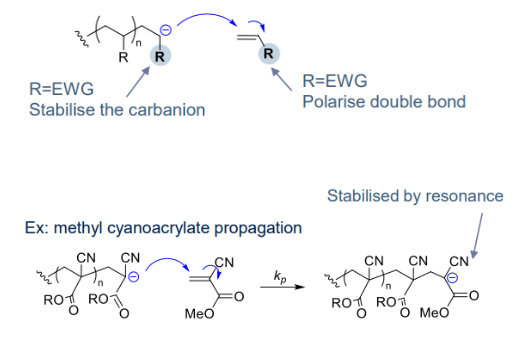
Favoured by EWG: via stabilised carbanions, resonance or inductive effects, and increased electrophilicity monomer (less e- rich, polarised double bond)¶ Transfer step
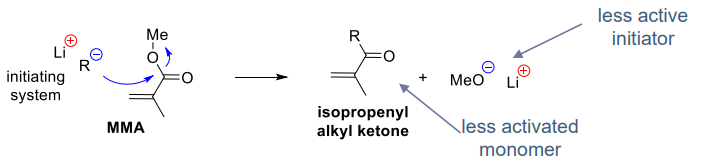
- Intermolecular nucleophilic substitution (SN) with the monomer (e.g. MMA)
- during propagation
- during initiation
- less active polymerisation and MMA / ketone copolymerisation - the alkyl ketone doesn't get polymersied much further
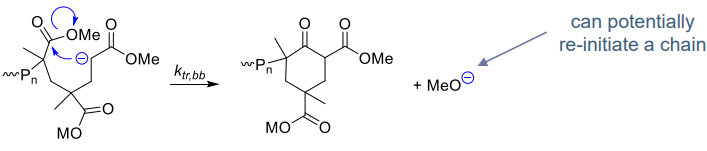
- Intramolecular nucleophilic substitution (SN) with the chain
- cyclisation via backbiting
¶ Termination step
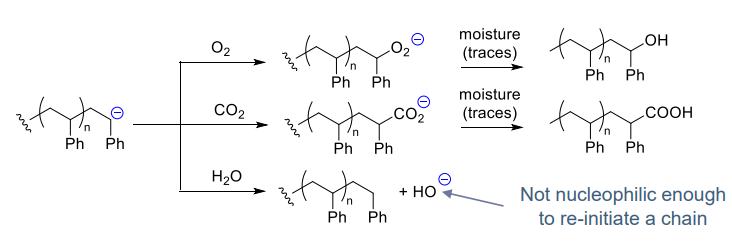
- Recations with (protic) impurities:
- water (or ROH) can be deliberately added to stop the polymerisation
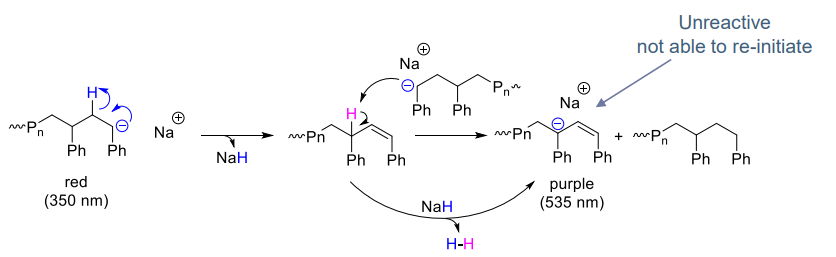
- Spontaneous termination:
- via hydride elimination
- Reduced at low temperature (< 0 ℃)
- Cation dependant: stability = K+> Na+ > Li+
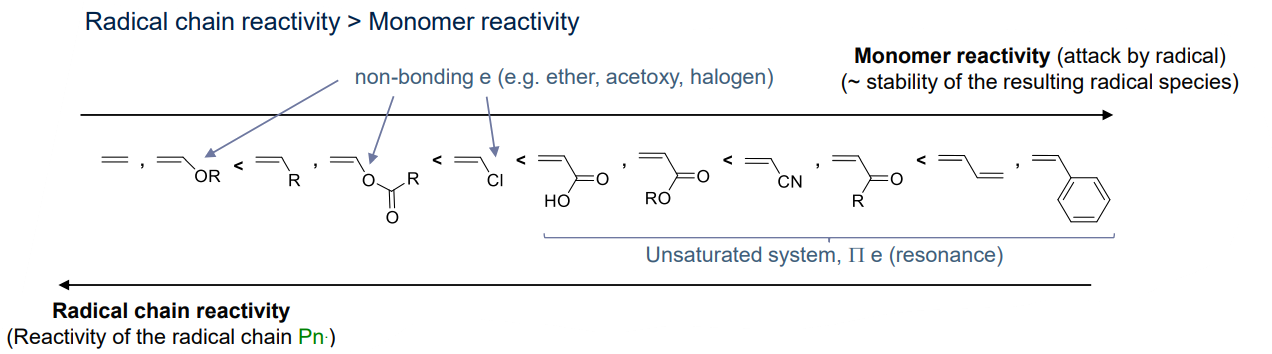
Radical Chain Polymerisations
Almost all substituents can stabilise radical species by delocalisation
- The nature of the substituent does not affect the ability to polymerize but it can affect the rate of i.e. copolymerisation
¶ Initiation step:
Controlling polymer molar mass and dispersity:
Good initiators
- readily available
- stable under ambient / redrigerated conditions
- controllable radical generation in "mild" conditions (e.g. T < 150 ℃)
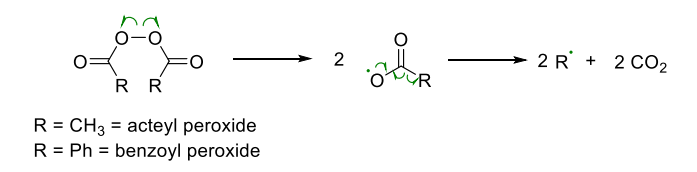
- Thermal initiators
- undergo thermal homolytic bond dissociation (gives rise to free radicals)
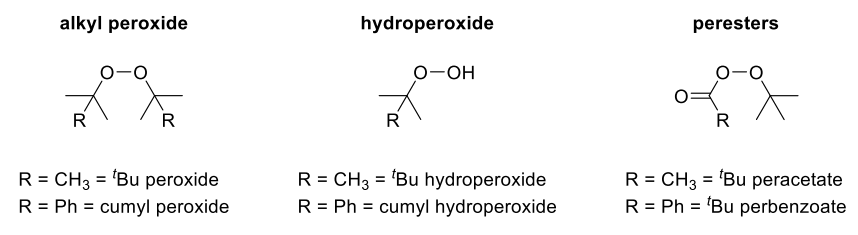
Examples:
- RO=OR peroxy compounds:
- RN=NR azo compounds: AIBN
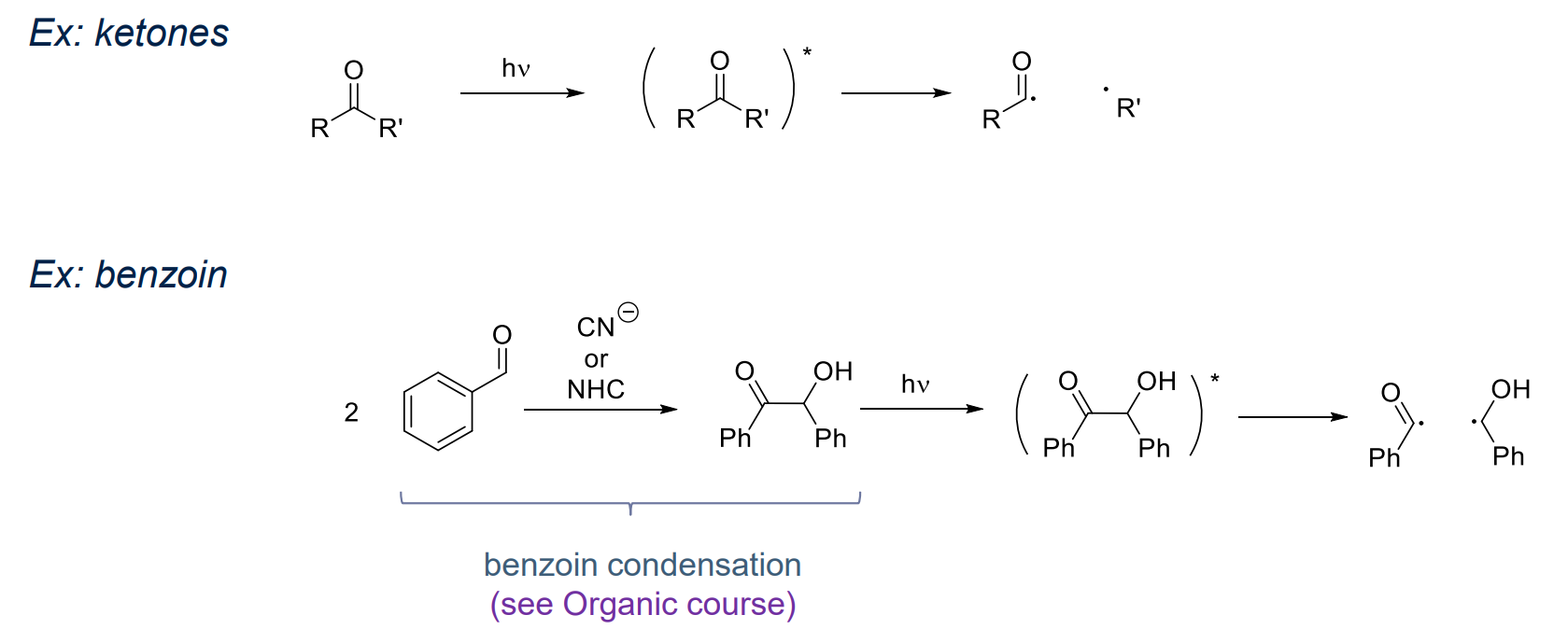
- Photo initiators
- Strong absorbance at given λ & high Φ for radical production required
Examples:
- same as thermal and redox initator which absorbs light
- via Norrish type 1 reaction: ketone and benzoin, and their derivatives
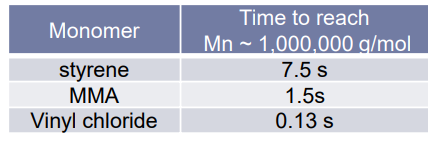
¶ Propagation step:
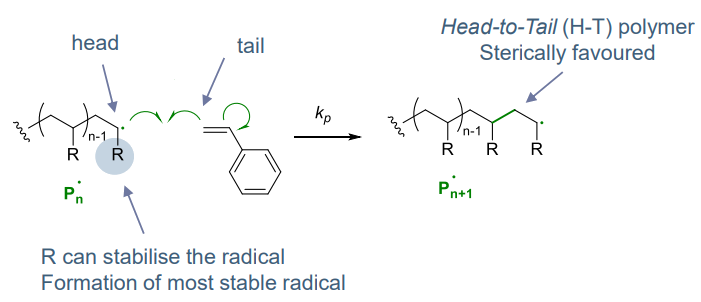
Reactivity:
Depends on radical stabilisation by the substituent via mesomeric & inductive effects
- head-to-tail (H-T) arrangement forms most stable radical
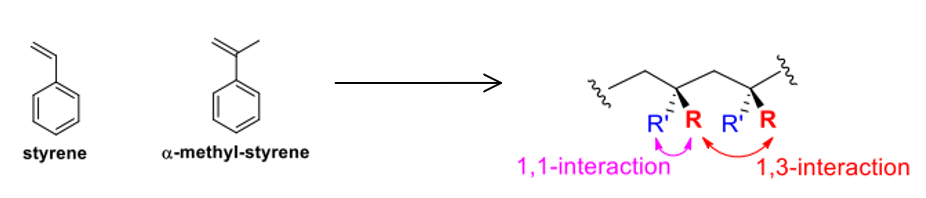
- sterically favoured over H-H (1,2 interaction instead of 1,3 / 1,1,)
- very fast step
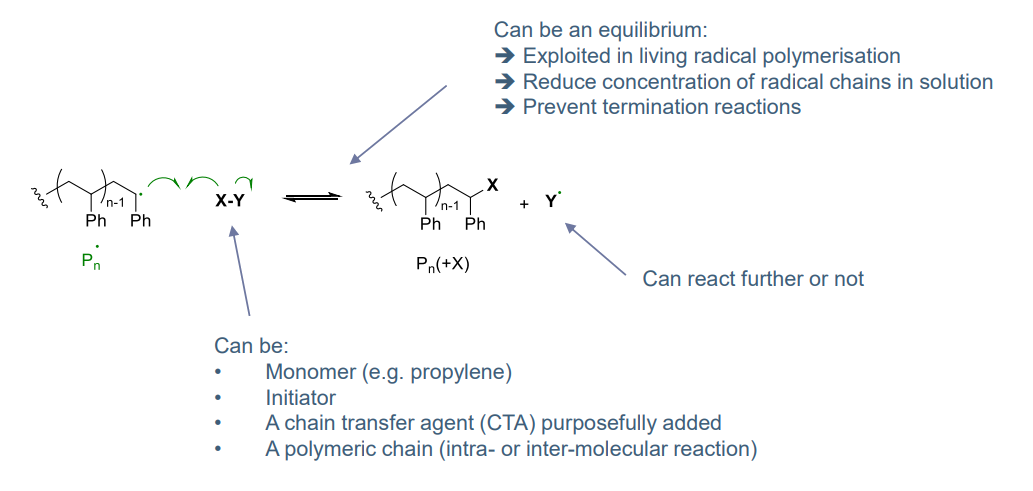
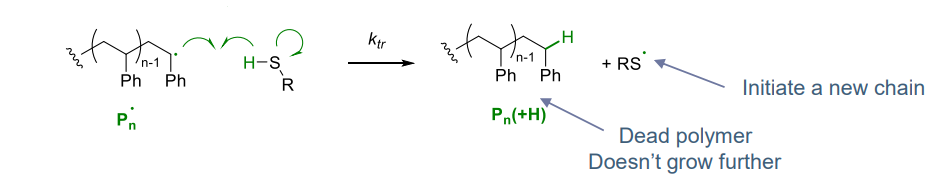
¶ Transfer step:
exchange of radicals
- can result in termination (e.g. resulting in alpha olefin)
- can reduce polymer mass (a good control)
- highly exploited for living radical polymerisation
examples:
- controlling molar mass
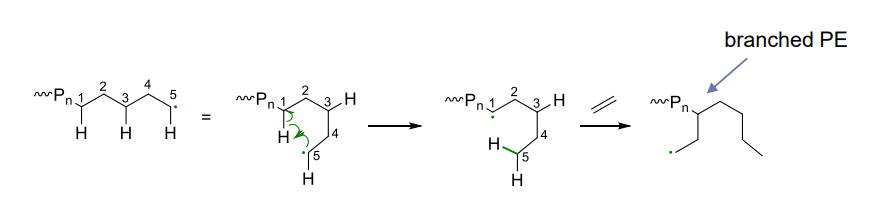
- inter / intra molecular leading to branches
- degree of branches controlled by conditions
- transfer to monomer
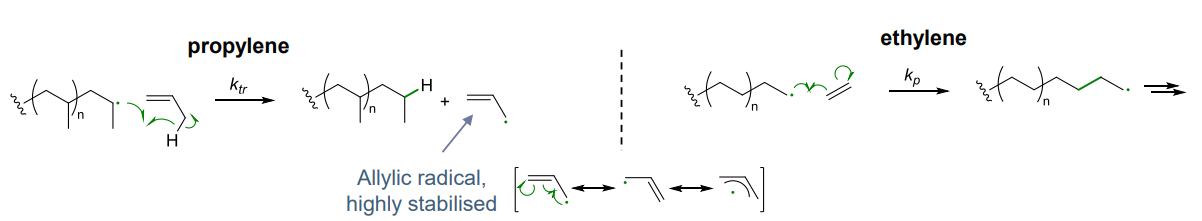
- Hydrogen radical transfer in alpha olefin e.g. propylene, requires ziegler-natta catalyst for polymerisation as the allyic radical is highly stabilised, not suitable for radical polymerisation
- no hydrogen radical transfer in ethylene so can be polymerised via radical mechanism - intramolecular, so branching can be observed
¶ Termination step:
Dead polymer generation
Combination (Route 1)
- two radical chains react with each other (H-H linkage) forming double molecular weight
- low radical concentration (10-6 ~ 10-9 M) → reduce coupling
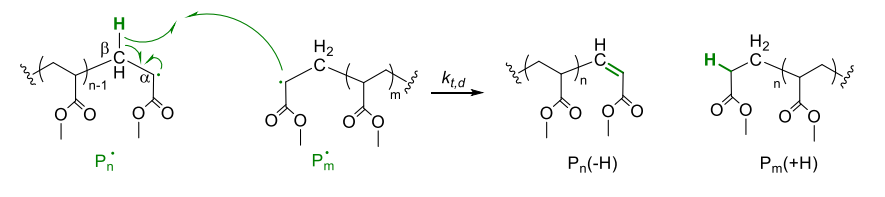
Disproportion (Route 2)
- hydrogen radical in beta position is transferred to other chain
- two chains are terminated with 2 different end groups
- no effect on molecular weight
Termination by transfer (Route 3)
- when the newly generated radical cannot initiate a new chain
- uses inhibitor for storgae/transport
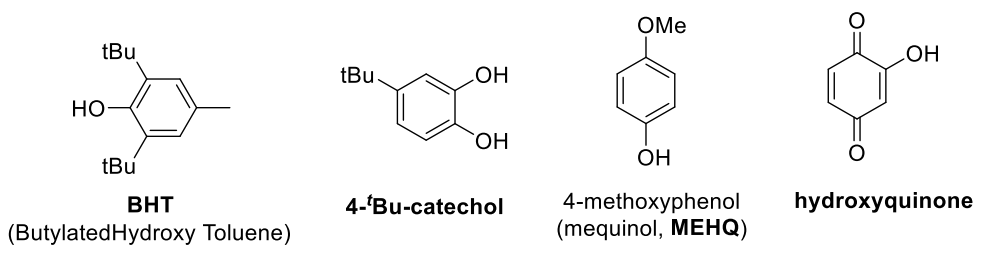
- pros: increase monomer shelf life, prevent polymerisation during transport, needs to be removed before polymerisation
¶ Polymer examples
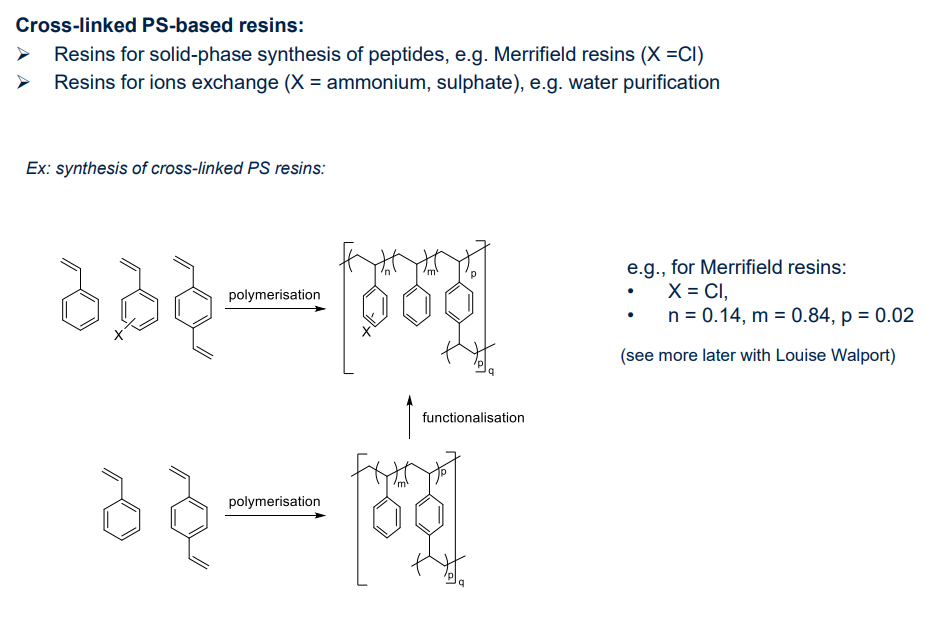
- polystyrene
- cross linked polymers
¶ More chain growth polymerisation
Apart from the three main chain-growth types, there are some subsets including coordination polymerisation and living polymerisation
¶ Coordination polymerisation and polyolefins
(similar to anionic polymerisation)
coordination spheres are controlled with using ligand(s) and metals, forming coordination catalysts
- High activity
- Less (or controlled) side-reactions (termination, transfer) by living polymerisation
- High molar masses, low dispersity
- Tacticity control (iso-, syndio-, hetero-)
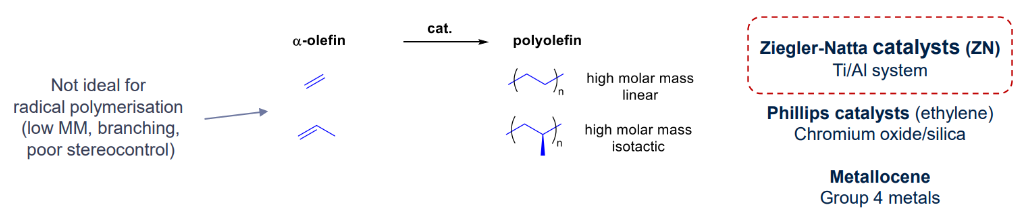
a-olefin polymerisation
Ring-Opening polymerisation (ROP)
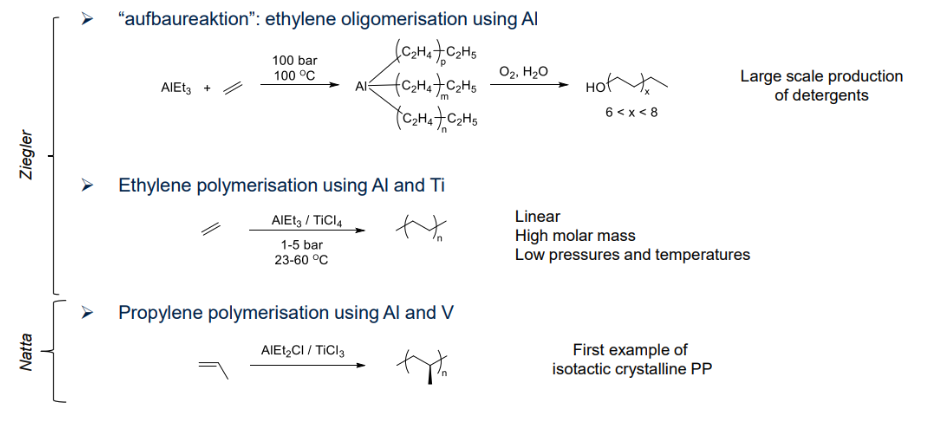
Ziegler and Natta catalysed reaction (coordination polymerisation)
Coordination catalysts for polypropylene
- ZN “catalysts” are still and mainly used for polyolefins production: Can produce up to 1000 kgpolymer/gcat
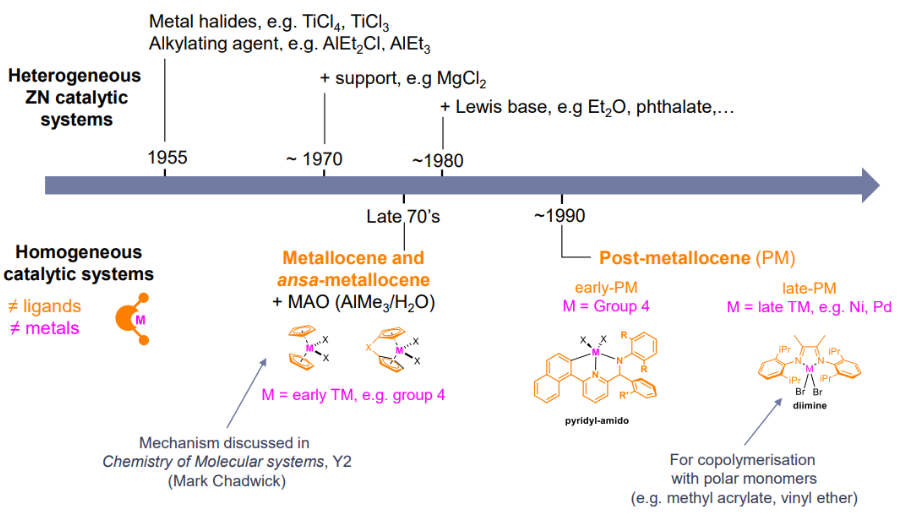
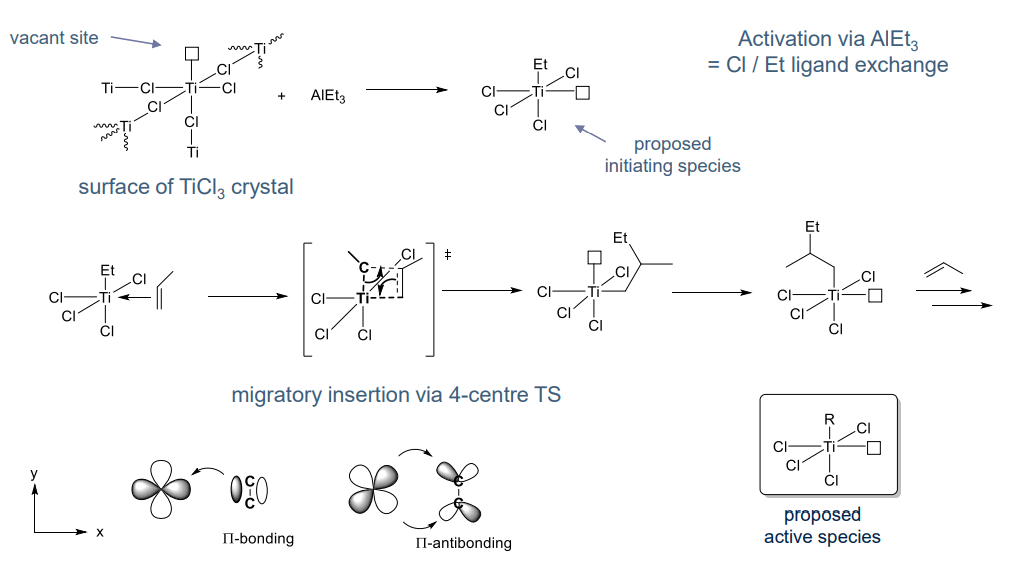
NON EXAMINABLE Heterogeneous ZN polymerisation mechanism:
- Relatively complex
- More difficult to characterise vs homogenous systems
- Involve Ti(III)Cl3 species
Types of polyethylene
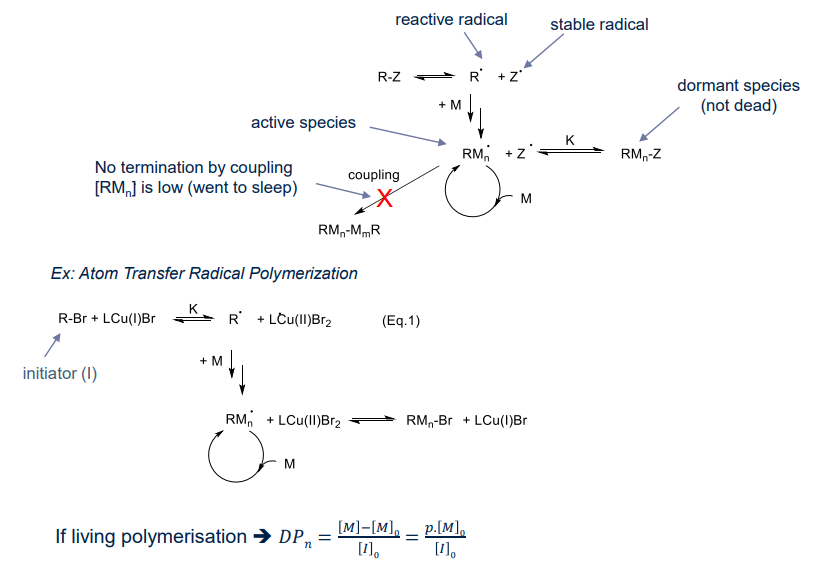
¶ Living (chain) Polymerisation
Living polymerisation ➔ no chain breaking reactions ➔ making transfer or termination REVERSIBLE and FAST
Chain breaking reactions are:
- difficult to predict polymer molar masses (how many chains are generated)
• difficult to predict dispersity → chain are generated at different instant during the polymerisation
• loss of end-group fidelity → chain-end reactivity (e.g. b-H transfer)Advantages of living polymerisation
Predictable polymer molar masses (DPn, Mn) based on conditions
- ratio of consumed monomer and number of chain
Narrow dispersity
- fast initiaion, all chain starts at the same time
End-group fidelity
- functionalisation of the polymer end-chain
- determined by MALDI-ToF
Access to block copolymers
- sequential addition of different monomers
Via different types of polymerisation:
- radical
- anionic and metal-catalysed
- cationic which are more difficult due to beta hydride transfer
Require no chain breaking reactions
- no impurities (e.g. promoting irreversible termination)
- reversible and fast transfer or termination reactions
Examples of living polymerisations
- Radial polymerisation:
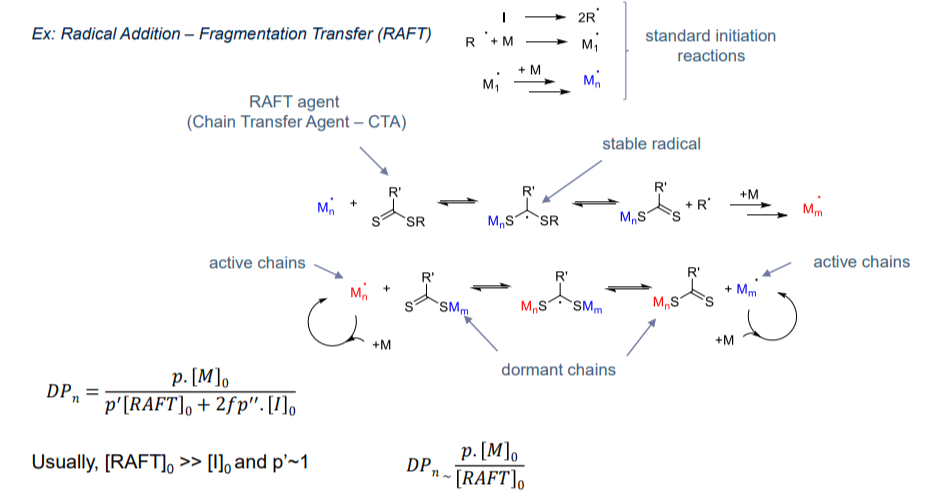
- ATRP & RAFT
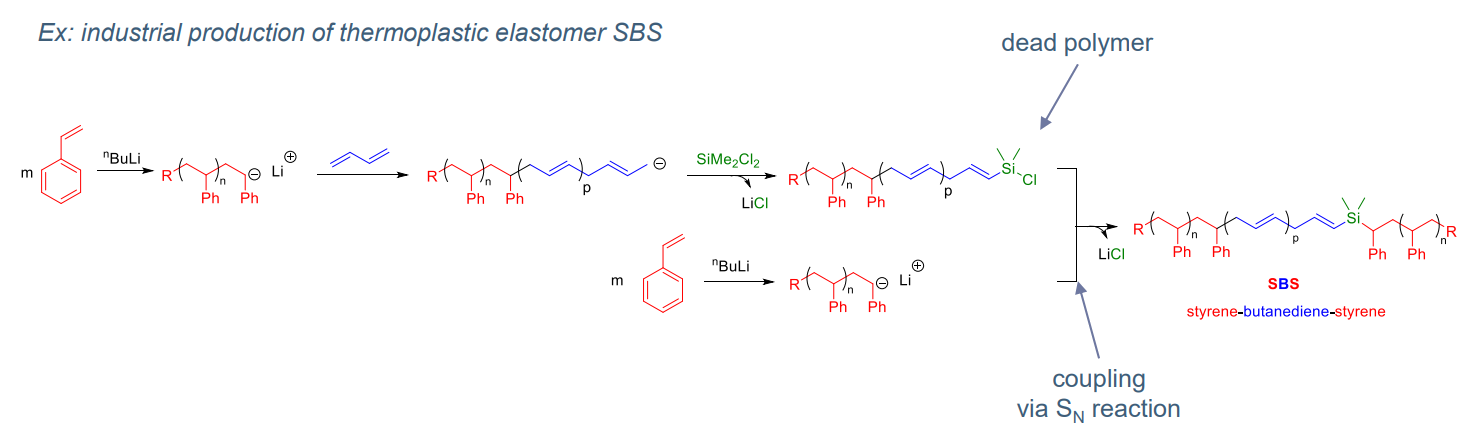
- Anionic polymerisation:
- Access to block copolymers
- Metal-catalysed Ring-opening polymerisation (ROP):
- Cyclic esters (e.g. lactides)
- Radical living polymerisation:
- To prevent termination by coupling → low concentration of active chain
- Reversible termination
- Reversible transfer
- Anionic living polymerisation
- “easier” to achieve than for radical or cationic
- No termination by coupling (contrary to radical polymerisation)
- Can be achieved using the right conditions:
- Choice of solvent
- Choice of counter-cation
- Temperature
- No impurities
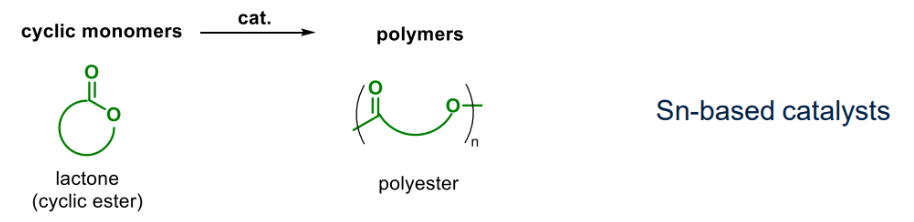
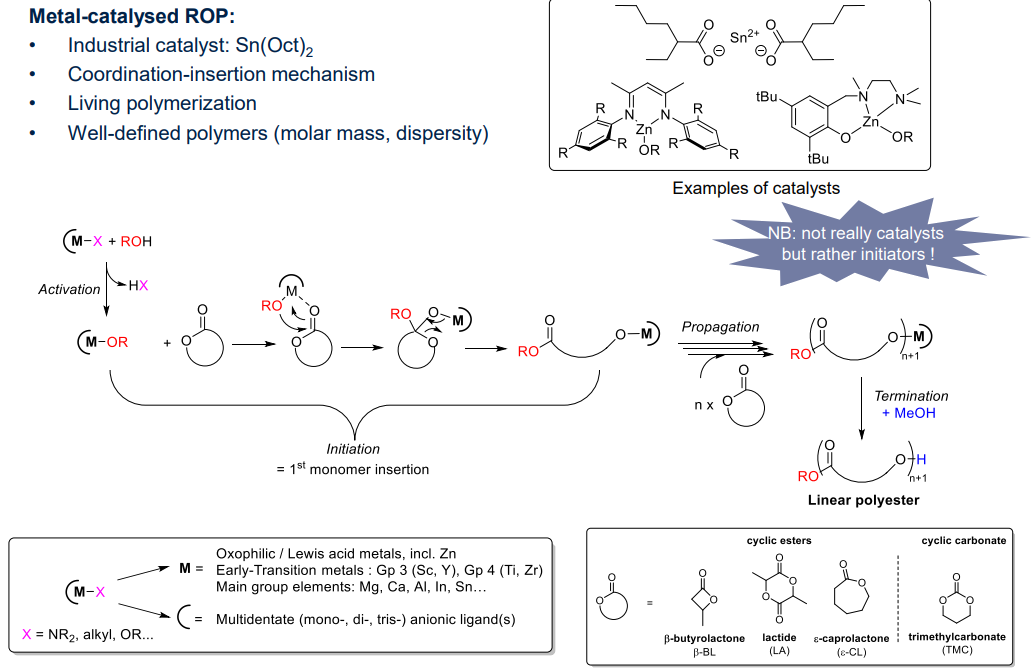
¶ Living Polymerisation (ROP)
A subset of living polymerisation
- A broad scope of cyclic monomers:
- ethers, amine, ester, carbonate...
- access to O-, N- or S-containing polymers
- Polymers obtained via (living) chain polymerisation:
- aninoic and metal catalysed
- catnionic, radical, enzymatic
- access to "sustainable" polymers
- monomers can be bio-based
- polymers can be (bio)degradable
Example of metal-catalysed ROP:
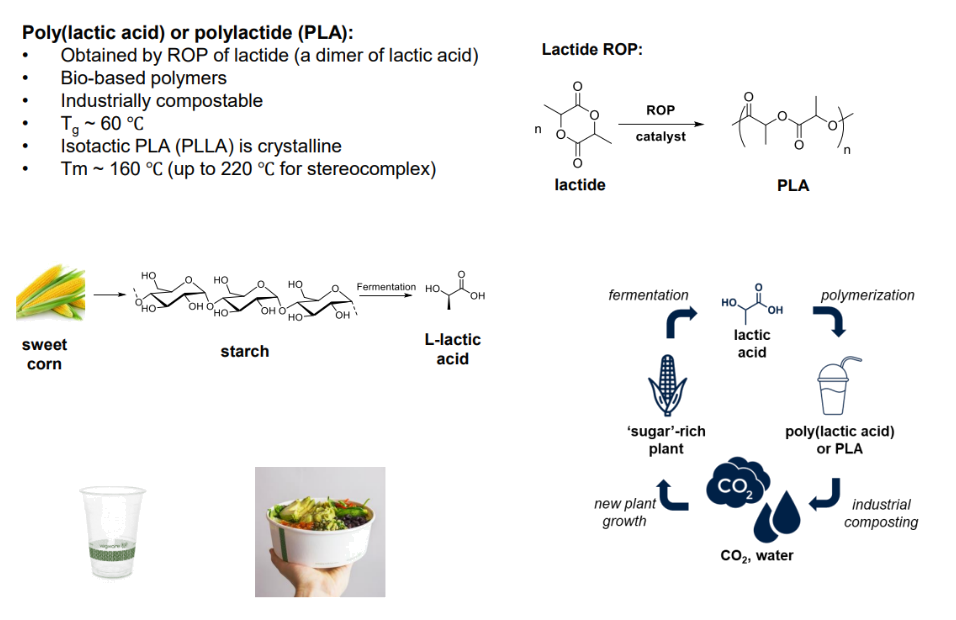
Example of ROP of cyclic esters: PLA & lactides
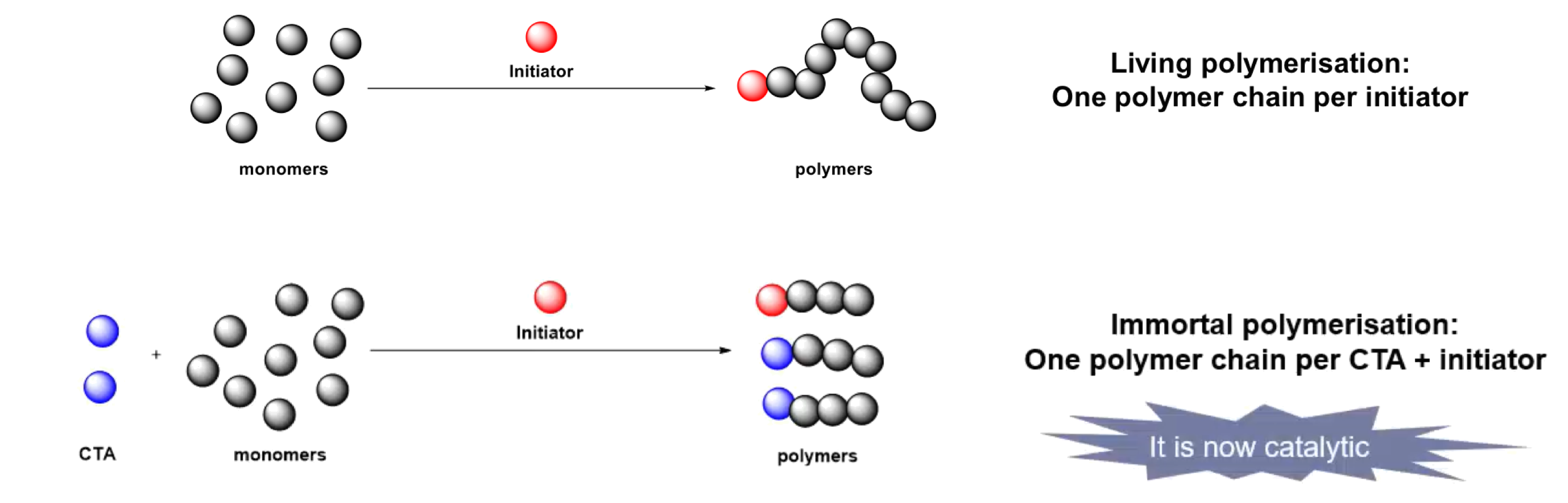
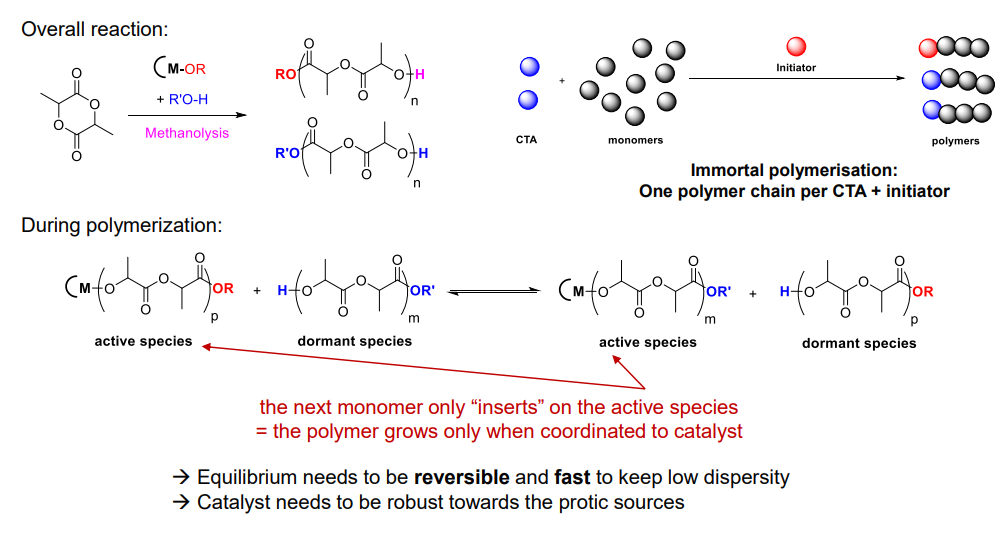
¶ Immortal polymerisation
- Well-controlled polymerization (Mn, PDI) in the presence of protic sources (typically alcohol) acting as a
chain-transfer agent (CTA)- Importance of the reversible reaction between active species and dormant species
- More polymer chains using same amount of monomers and catalysts
¶ Polymerisation feasibility
Consider the following factors
- Themodynamics
- If ΔG is negative, the process is thermodynamically favourable under standard conditions.
- ΔH < 0: from pi to sigma bonds
- ΔS < 0: from free monomers to “in-chain” monomers
- Kinetics
- of reasonable rate
- fitting specific T, Conc condition of product
- using catalsysts i.e. Ziegler-Natta for alpha olefins
- Monomer structure
(see more in ring opening polymerisation ROP where ΔS less affected)
- Consider differences from monomers to polymers:
- resonance stabilisation, will monomer lose conjugation?
- steric constraints: bond angles, deformation e.g. 1,1 and 1,3 creates steric constraints so a-methylstyrene is not (enthalpically) favourable
- non-covalent interactions: hydrogen bonding, dipole interactions etc.
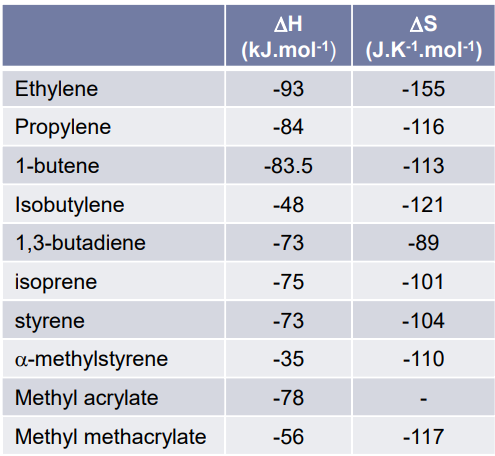
Table showing polymerisation enthalpy & entropy values at 25℃ of monomers containing C=C
¶ Polymerisation rate
kp is independent of polymer chain lenght
¶ Kinetics
¶ Living polymerisation
testing ignore this > testing