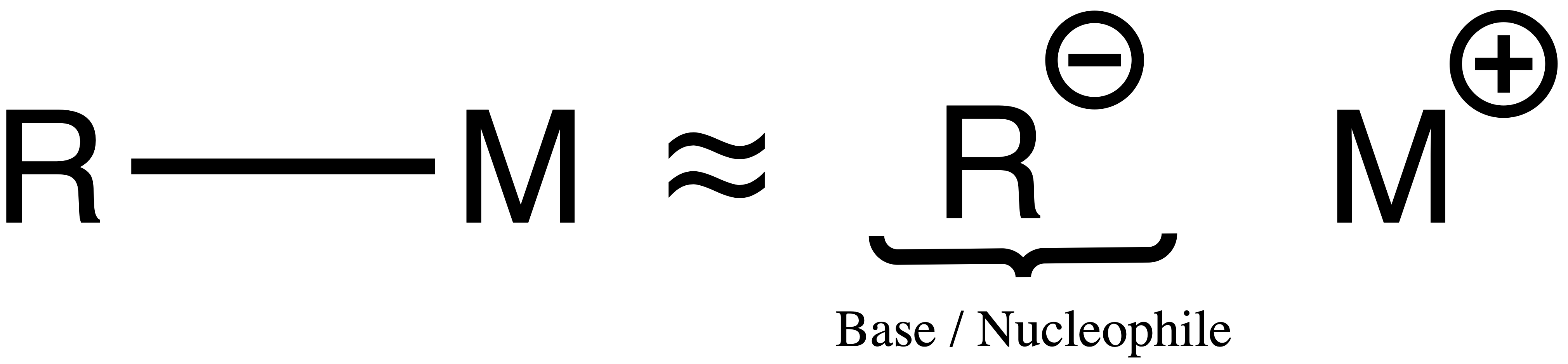Organometallics behave like nucleophile and bases
- A helpful way to think of them is to imagine them as carbanions
- The negatively charged carbon can then act as a nucleophile or a base or both
¶ General Reactivity Trends of R groups in R-M
In general, the reactivity of an organometallic is related to its
- The more basic ( The higher the ) the carbanion is, the more reactive it is
- More reactive means that it acts as a stronger nucleophile and base
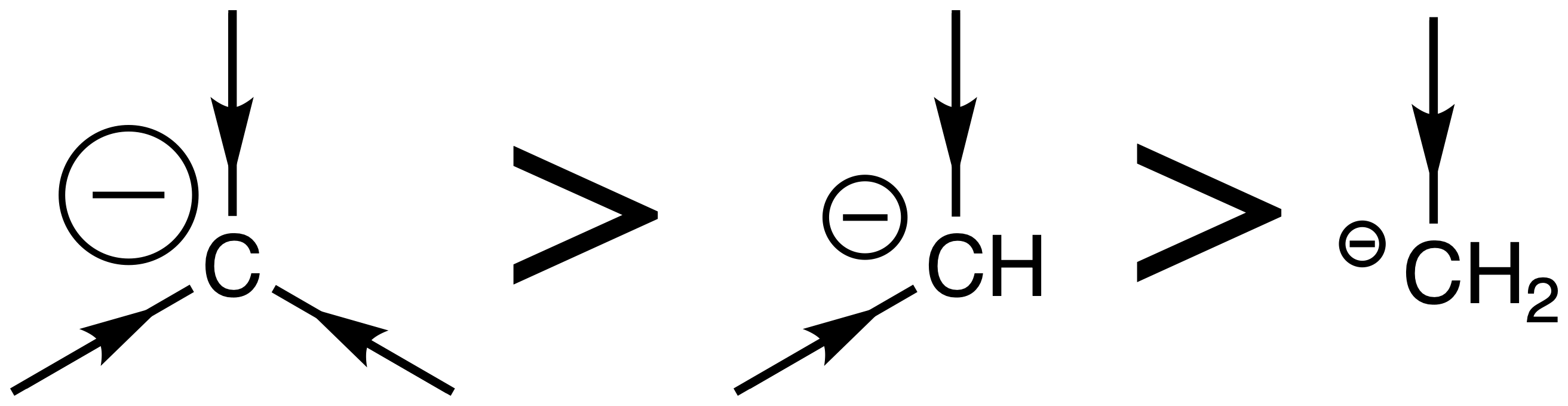
The pKaH of the carbanion is affected by 2 factors
- The degree of substitution :
- The hybridization of the carbon that is bearing the negative charge
- The conjugation of the carbanion
The higher the degree of substitution, the more reactive it is
- This is because the alkyl groups are donating electron density towards the carbanion
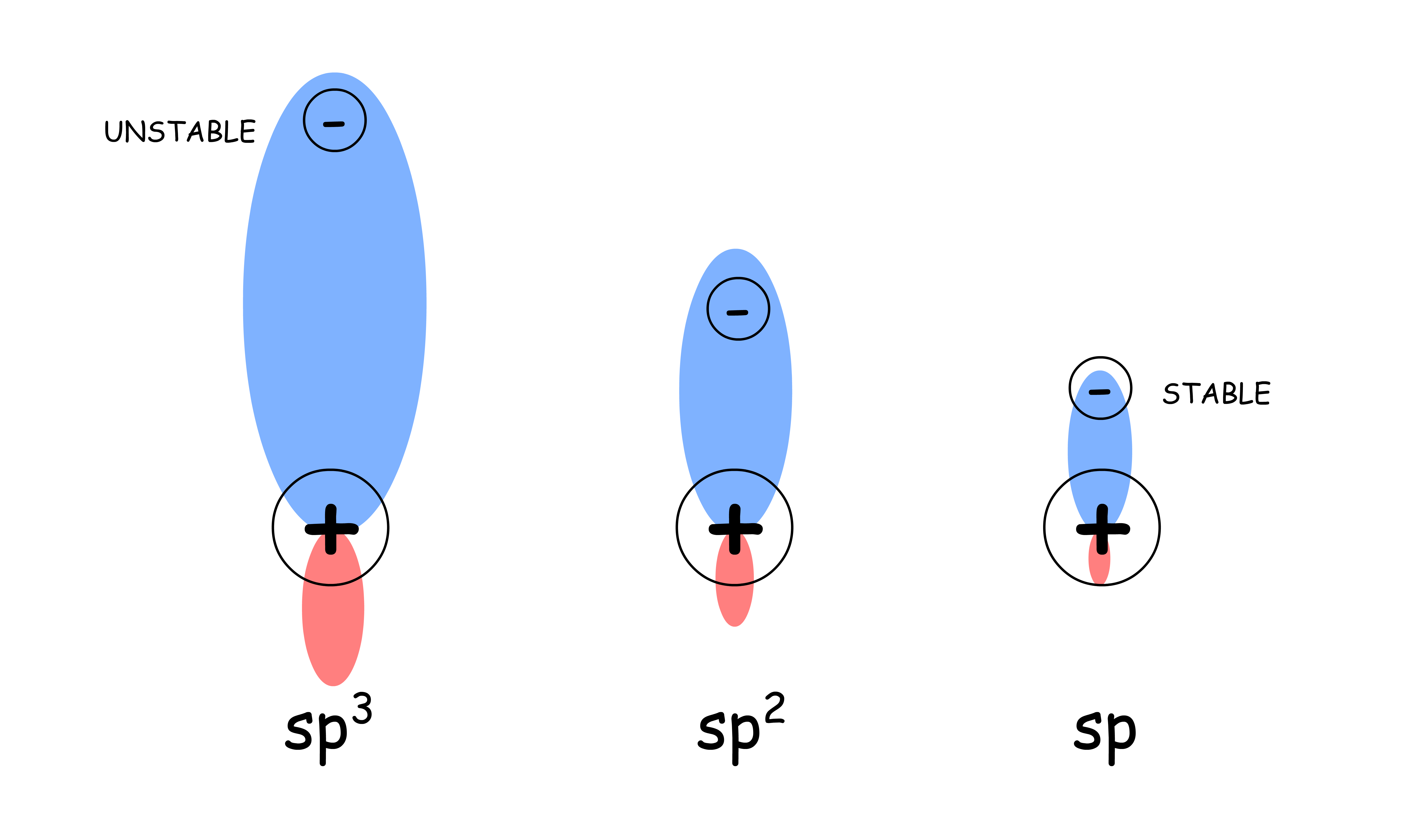
The less s character the carbanion has, the more reactive it is
- A higher s character means that the orbital is more contracted (closer to the positively charged nucleus), which helps stabilize the negative charge, making it less reactive
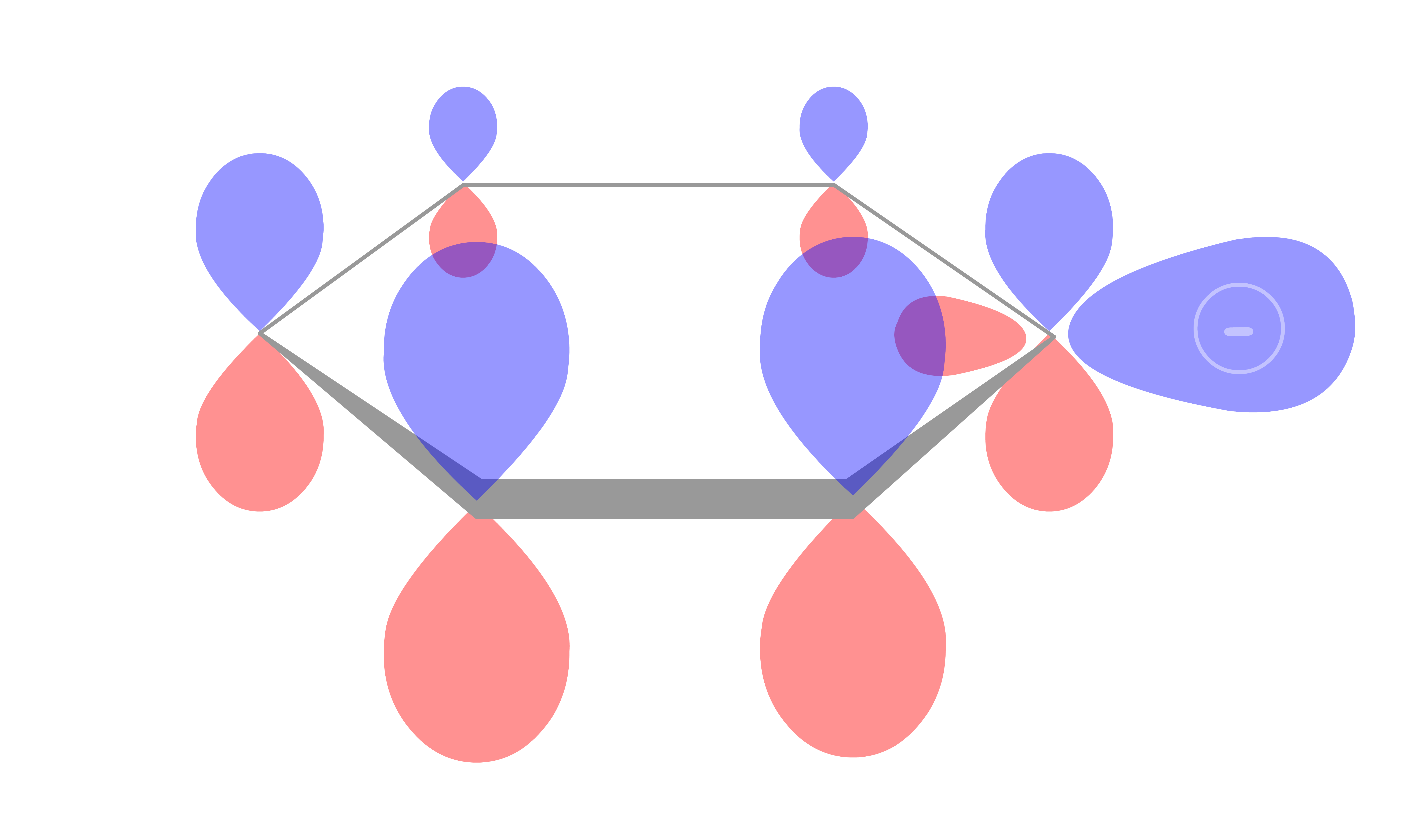
If the negative charge is conjugated, it will be less reactive
- The conjugation helps stabilize the negative charge, making it less reactive
- However, note that the negative charge may not necessarily be stabilized by conjugation simply because the carbanion is in a conjugated system.
These kind of situations usually happen when the negative charge sits on an orbital that is orthogonal to the conjugated system
¶ Selectivity from different Metals
¶ Organocerium
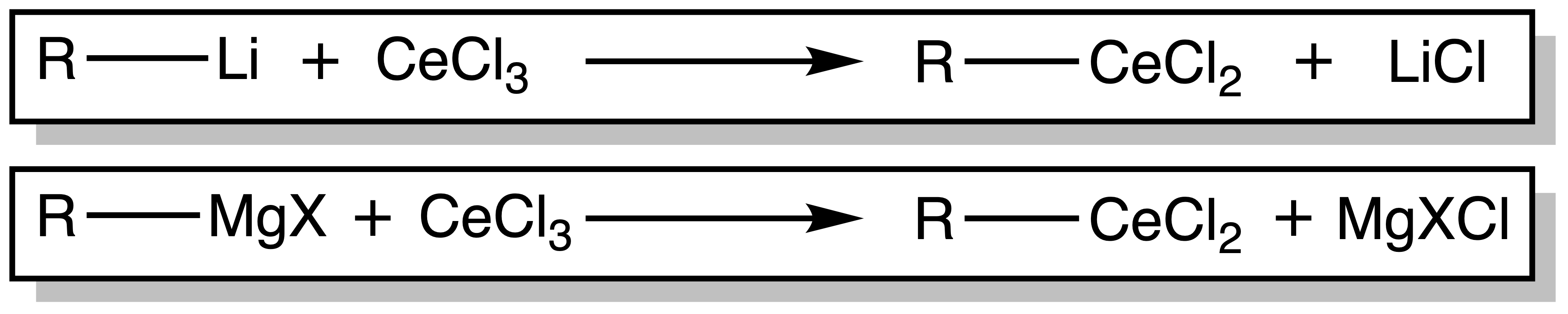
Cerium as a hard nucleophile
- Cerium (Ce3+) is a very hard specices, so it has a strong affinity for hard anion ( e.g. O , N )
- Reaction of R–Li or R–MgX and anhydrous CeCl3 affords organoceriums ‘R–CeCl2’
- The R–Ce bond in R-CeCl2 is very polar ( STRONG nucleophile ) but NON-BASIC!!!!
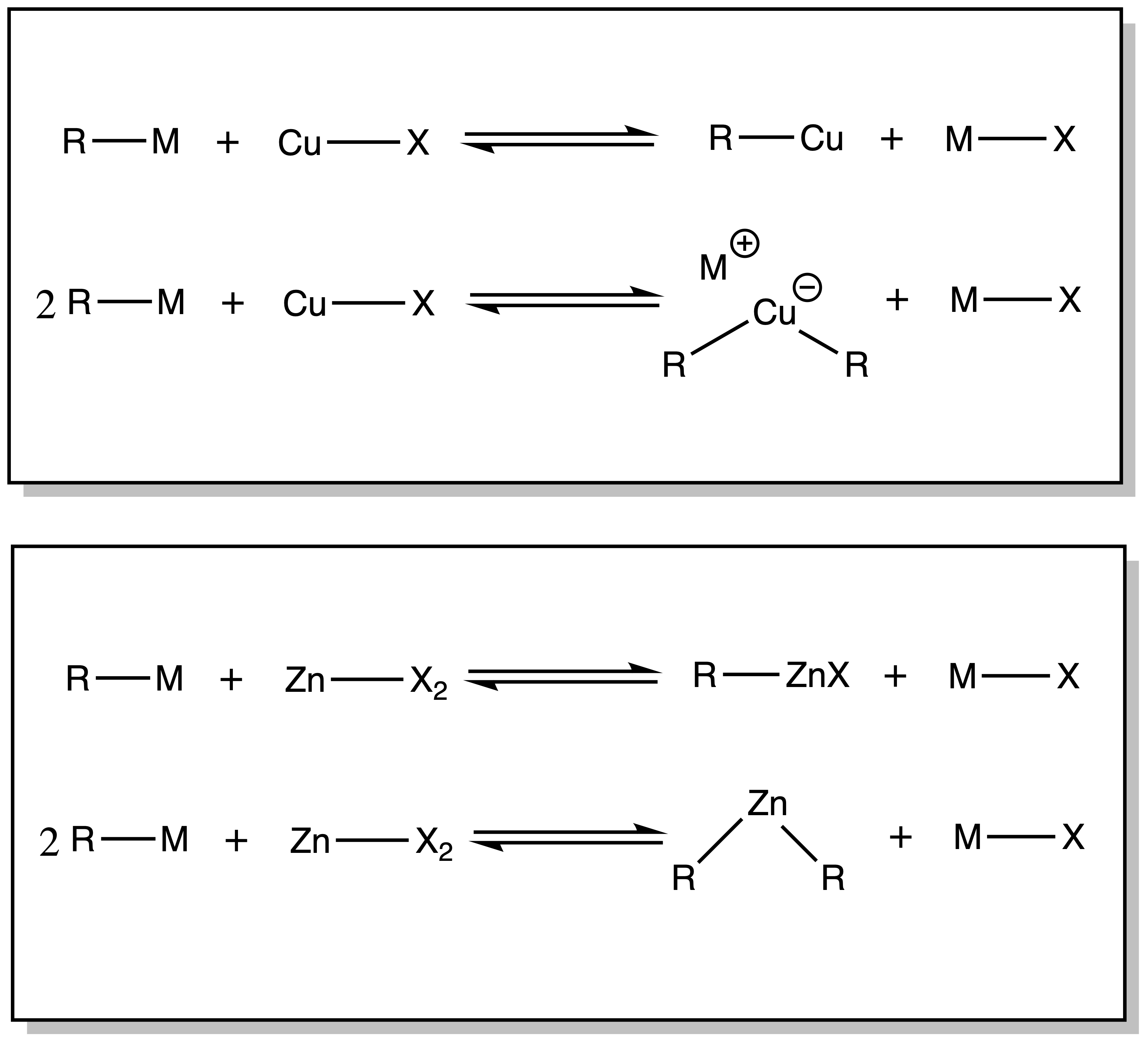
¶ Organocuperates
Copper as a softer nucleophile
- R-Cu is much more covalent than R-Li/R-Mg
- R–Cu reagents only undergo reaction with strong electrophiles
- Organocuprates have a significantly higher charge, so they are more reactive than R-Cu. Hence, it is an excellent SOFT nucleophilic source of R
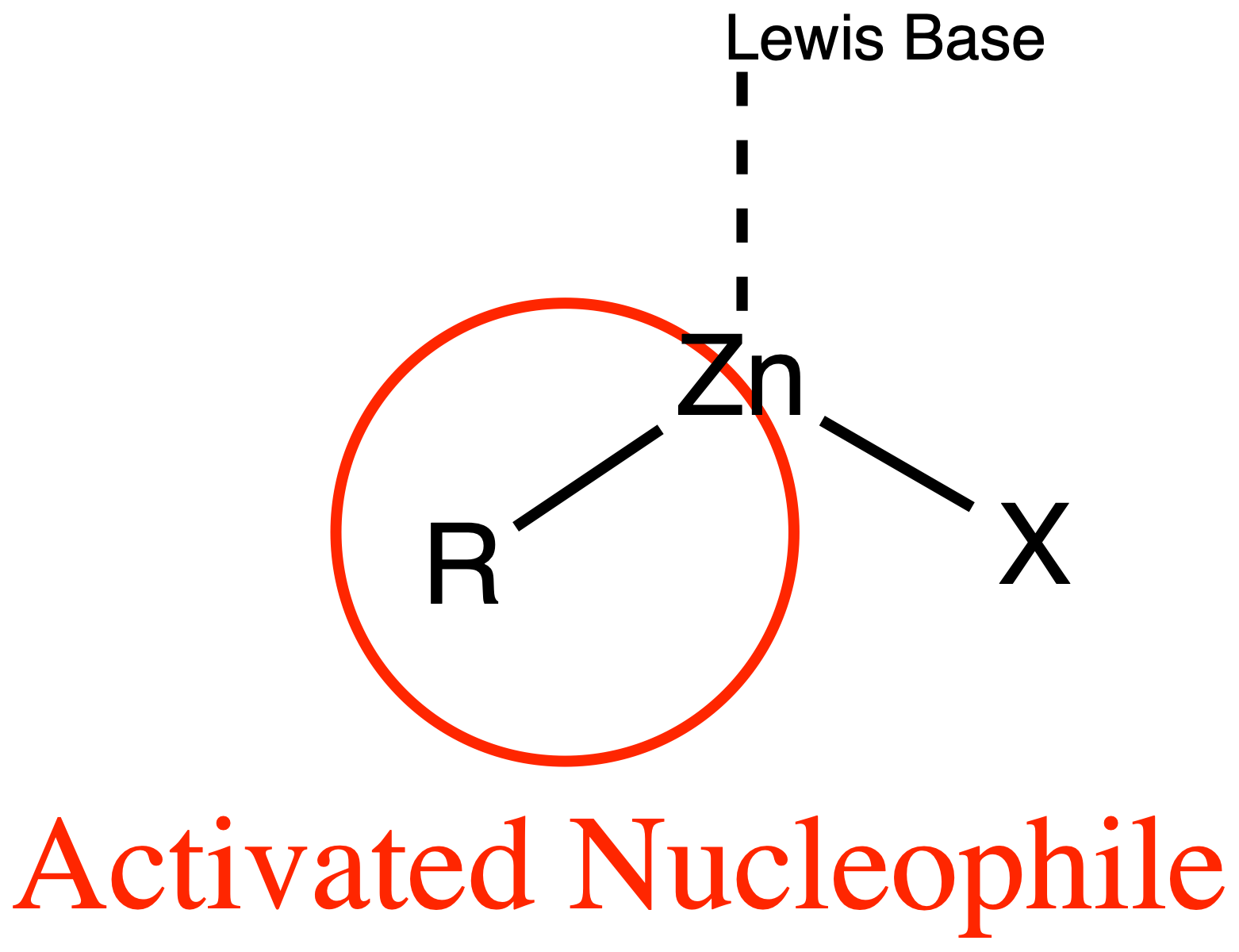
¶ Organozinc
Organozinc reagents are less reactive, more selective
- Considerably less nucleophilic than RLi, RMgX, reagents
Organozinc reagents do not readily add to typical organic electrophiles without additional ‘activation’ :
- Use of Lewis acid to enhance electrophilicity of substrate
- Use of Lewis base to bind R–ZnX to enhance nucleophilicity of R-Zn bond
- Note that the activating lewis acid or lewis base can be chiral, leading to an assymetric addition reaction
¶ Synthesis of Organometallic Reagents
¶ Direct Synthesis
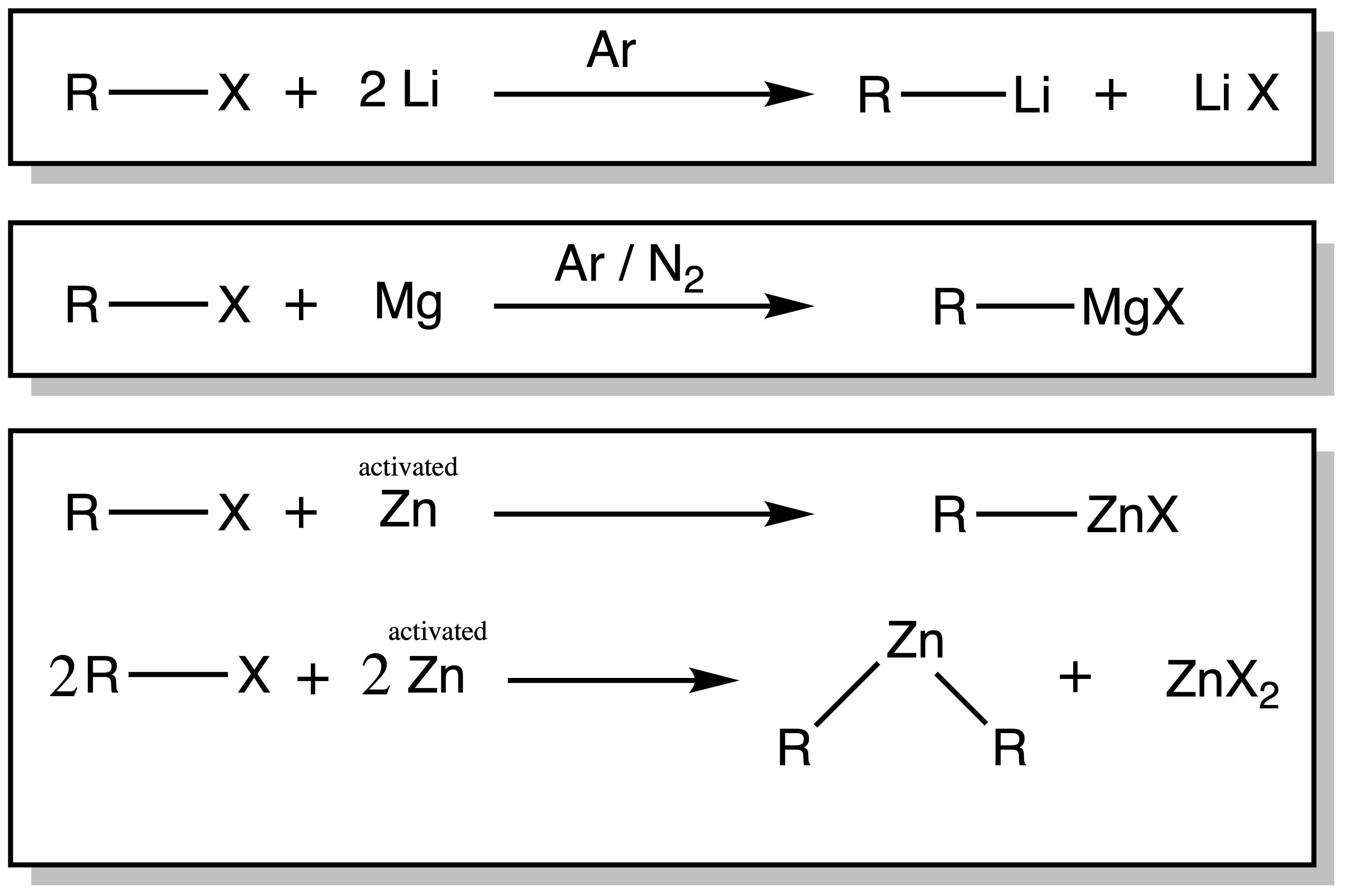
Organolithium and Grignard reagents can be produced via Direct Synthesis
- Must use anhydrous solvent and inert atmosphere (Note that lithium reacts with nitrogen)
- R–Li usually in hydrocarbon solvent, while Grignards require ethers
- Side-product R-R (Wurtz coupling) can be problematic for R = allyl/benzyl
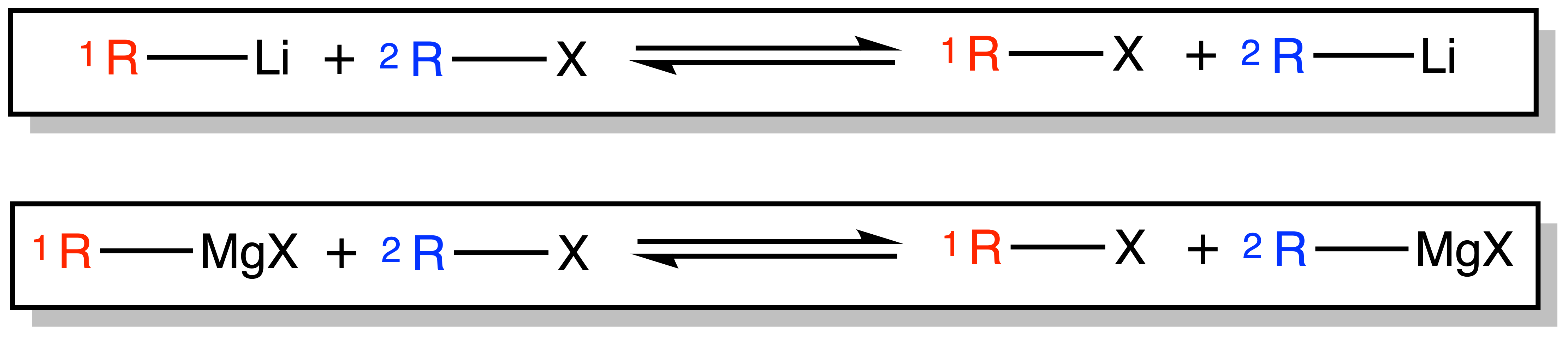
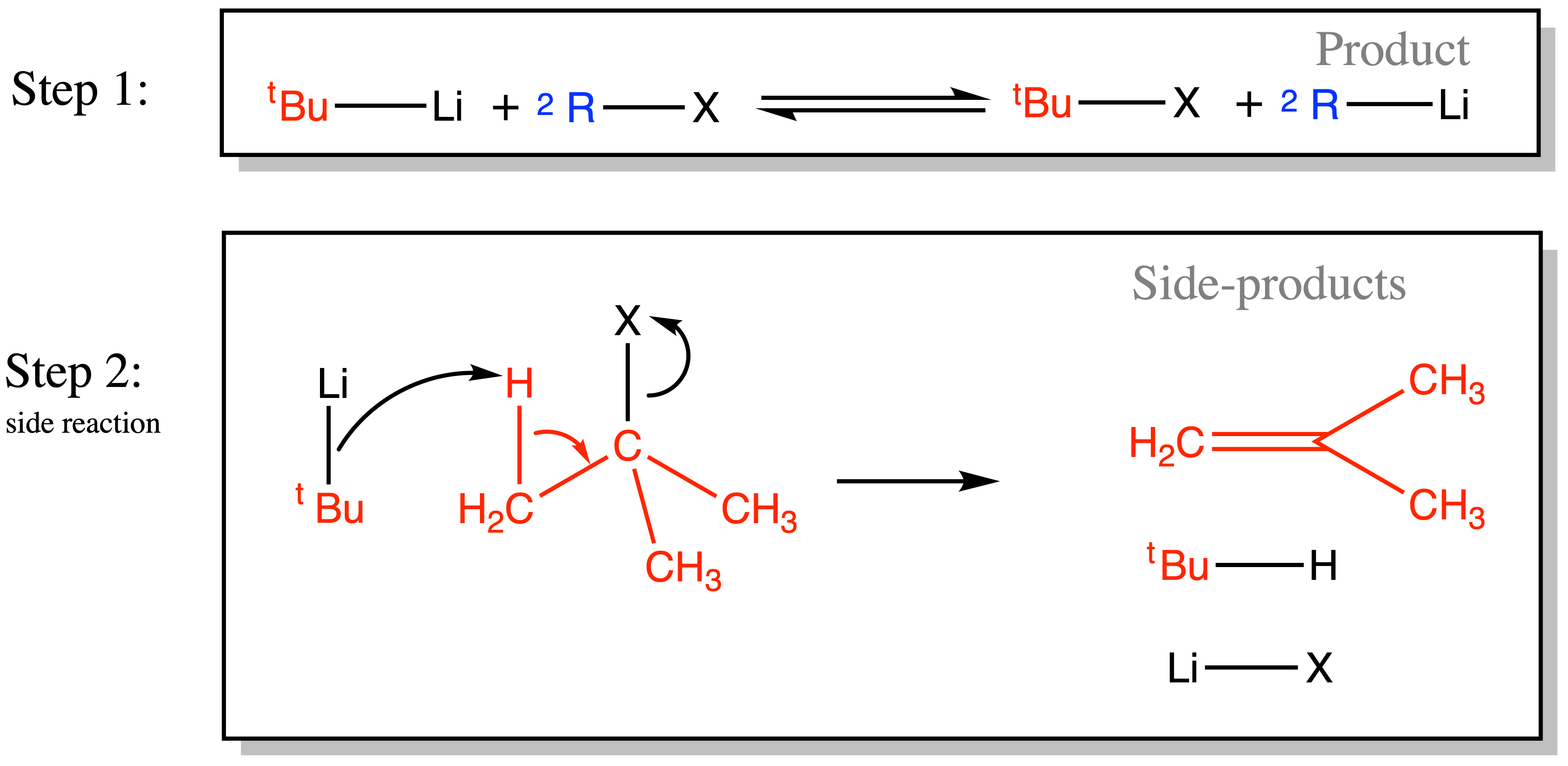
¶ Metal Halogen Exchange
Organolithium and Grignard reagents can be produced via Metal Halogen Exchange
- The reaction is in an equilibrium, but the equilibrium will lie towards the side of the product when
is a lot more basic than
( the pKaH of>> the pKaH of
)
- Note that if
, then we will need two equivalents of
–Li to produce one equivalent of
–Li
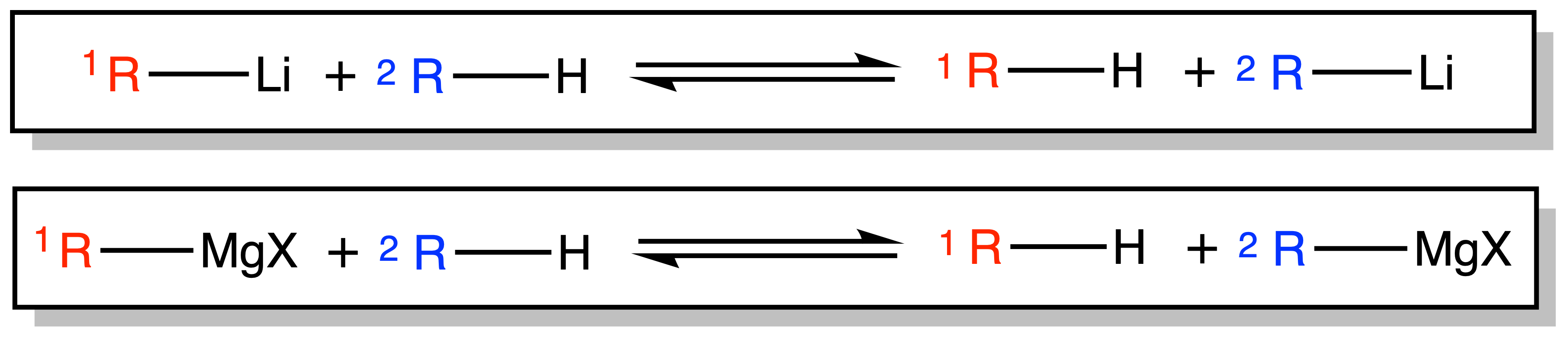
¶ Deprotonation
Organolithium and Grignard reagents can be produced via Deprotonation
- The reaction is in an equilibrium, but the equilibrium will lie towards the side of the product when
is a lot more basic than
( the pKaH of>>>> the pKaH of
)
Copper and Zinc reagents can be synthesized via Transmetallation reaction
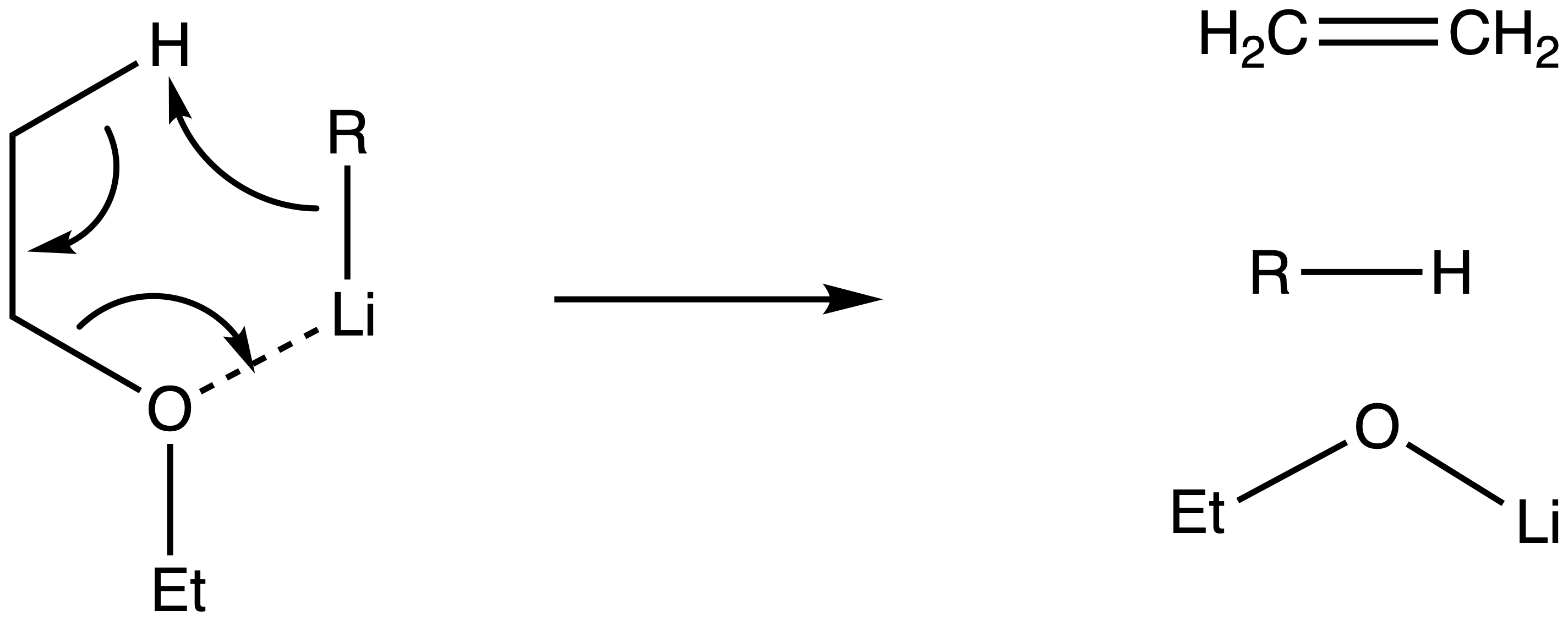
¶ Stability of Organolithium
R–Li species are highly reactive due to their high bond polarity
- In fact, it is so reactive that it will decompose before the reaction even starts
Organolithium can decompose by reacting with the ether solvent
- Ethers coordinate to Li, which increases the reactivity of R–Li
- An increased reactivity also implies an increased basicity, which causes the carbanion to deprotonate the hydrogen on the ether solvent, thus initiating an elimination reaction
- For this reason, the R-Li is more stable when is less basic (which is why only Me-Li is stable in Et2O)
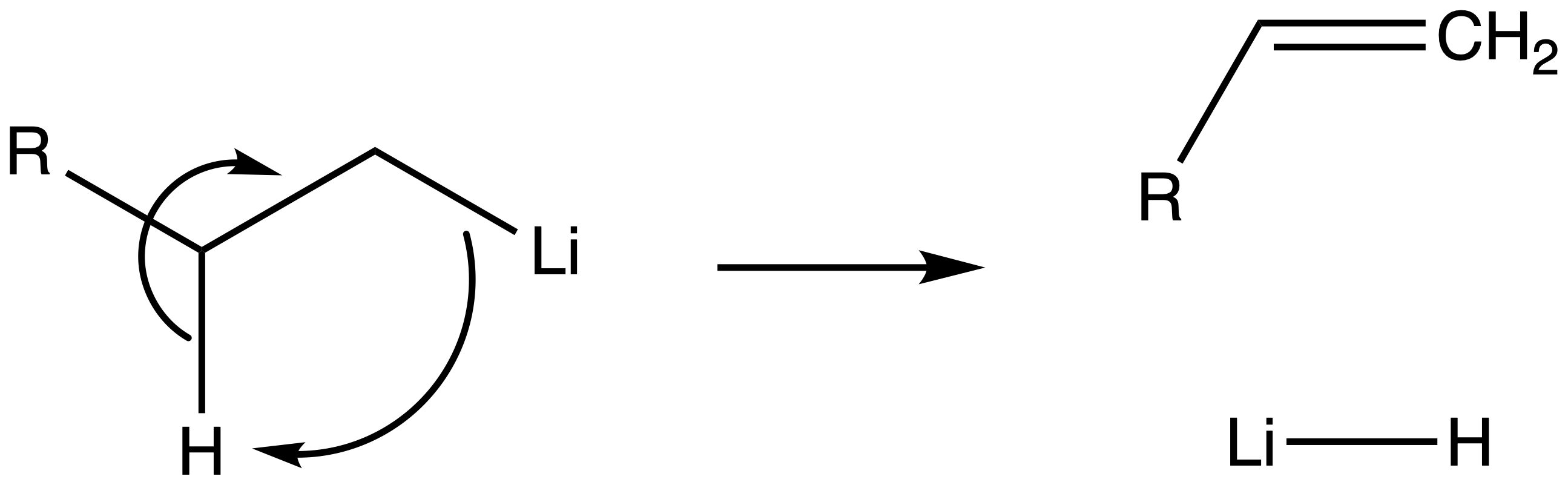
Organolithium can decompoe via an intramolecular reaction above room temperature
- This is called the -hydride elimination