¶ Inorganic Mechanism
Inorganic mechanism are investigated at two levels
- Stochiometric Mechanism
- Intimate Mechanism
Rates of reactions are used elucidate the mechanism
- They give us details of the activation process and rate determining step
¶ Stoichiometric Mechanisms
Stoichiometric mechanisms provide a description of the sequence of elementary steps involved in a reaction
- These steps outline the individual molecular events that lead to the overall transformation
- By examining the stoichiometric mechanism, we can gain insights into the reaction pathway and the intermediates formed during the process.
There are three main types of Stoichiometric Mechanisms
- Associative
- Dissociative
- Interchange
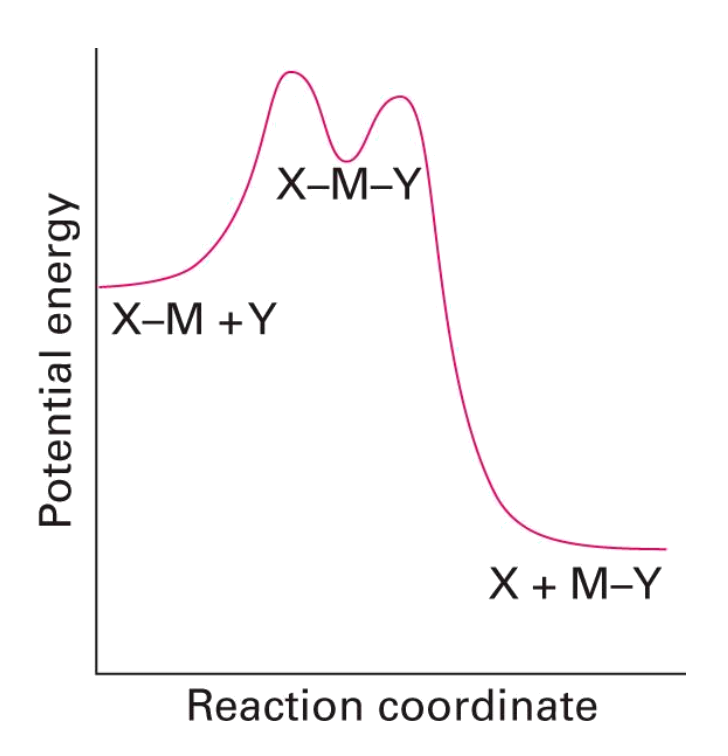
Associative
- M-Y bond forms before M-X bond breaks
- Intermediate with an increased coordination number is formed. Rate depends on both [MLnX] and [Y]
- Reaction classed as associative if we can ‘see’ the intermediate complex with an increased coordination number
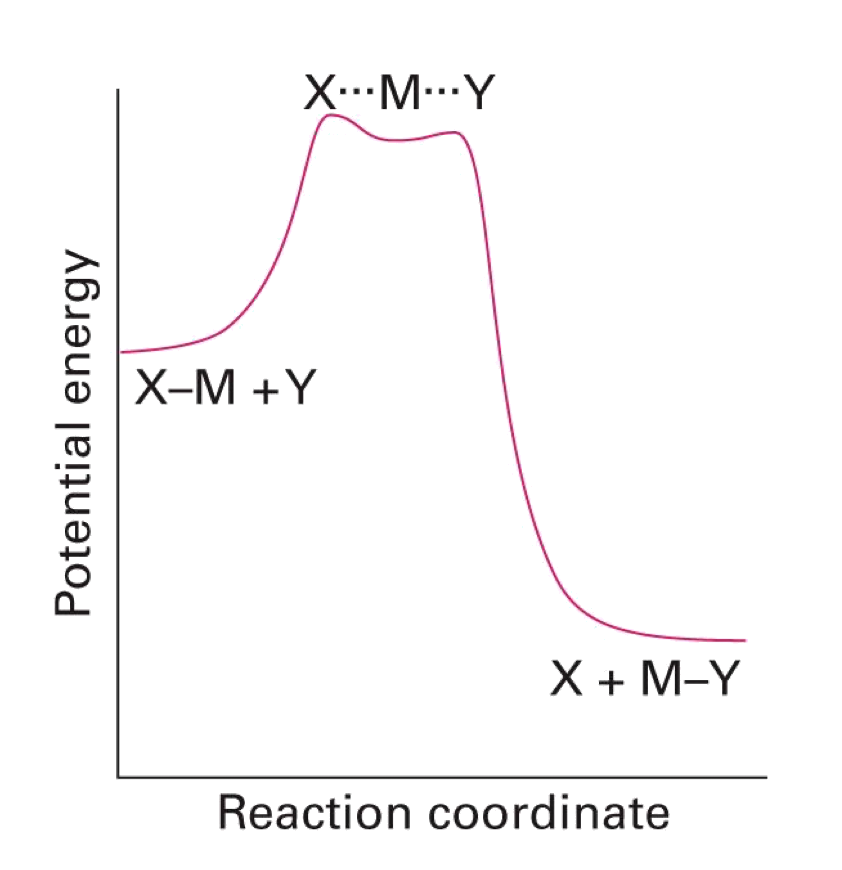
Dissociative
- M-X bond breaks before M-Y bond forms
- Intermediate with a decreased coordination number is formed. Rate depends on both [MLnX], but not [Y]
- Reaction classed as dissociative if we can ‘see’ the intermediate complex with a decreased coordination number
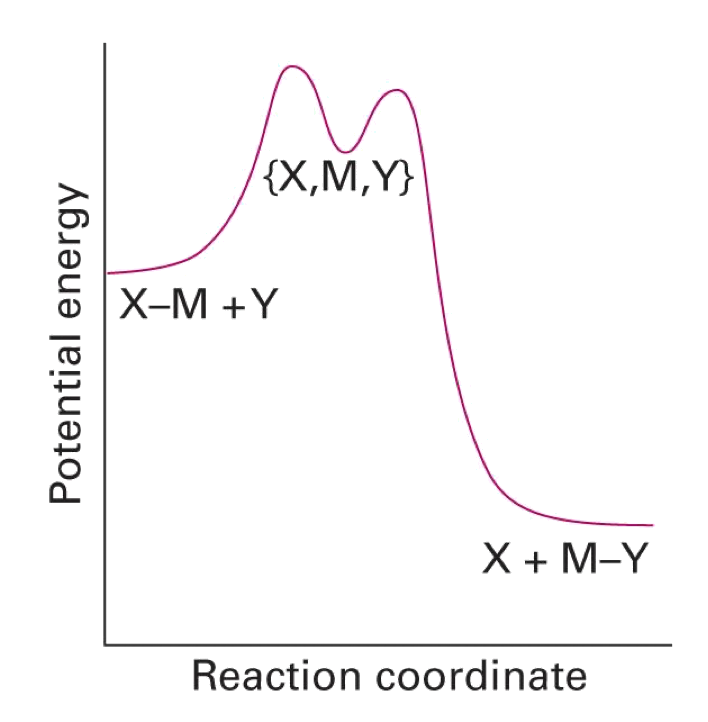
Interchange
- M-X bond breaking and M-Y bond formation is at the same time
- We expect the rate to depend on both [ML_nX] and [Y]
- Reaction classed as interchange if we cannot ‘see’ the intermediate complex
¶ Intimate Mechanisms
Intimate mechanisms provide a more detailed understanding of the formation of the transition state
- These mechanisms focus on the microscopic events that occur during an individual step of the reaction, shedding light on the molecular changes and interactions involved
- The intimate mechanism of a reaction is particularly important because it often corresponds to the rate-determining step (RDS)
Two main types of intimate mechanisms can be classified based on the nature of the transition state
- Associative Activation
- Dissociative Activation
Associative Activation
In an associative activation mechanism, the rate of formation of the activated complex depends on the nature of the incoming ligand
- The activated complex in this case involves significant bonding to the incoming ligand, which plays a crucial role in stabilizing the transition state
- The presence and nature of the incoming ligand can greatly influence the rate of the reaction and the overall reaction pathway.
- due to the increased order of activated complex
Dissociative Activation
In a dissociative activation mechanism involves the breaking of a bond, so the rate of formation of the activated complex does not depend on the nature of the incoming ligand
- The rate of formation of the activated complex in this case is controlled by the M-X bond breaking process
- due to the increased order of activated complex
¶ Inert and Labile
The terms ‘inert and ‘labile’ relate to the length of time a thermodynamically unstable complex will ‘survive’, and time taken to reach the equilibrium position,
Factors that control whether a complex is inert or labile
- Small ions are less labile, as stronger M-L bonds and sterically ‘restricted’ at metal centre
- Complexes with no CFSE or chelate effects tend to be most labile.
¶ Substitution in Square Planar Complexes
For most square planar complexes rate depends on both concentration of complex and incoming ligand
- The data is not simple first or second order kinetics
¶ Variation of incoming group Y on K2 path
rate will be faster for nucleophilic entering groups
- i.e. soft, polarisable ligands that bind more strongly to the soft Pt(II) (HSAB) have the largest rate constants
¶ Variation of leaving group X on K2 path
Provides information on the extent of bond formation in a complex intermediate
- Generally, order of leaving group is reverse of the entering group.
- The effect of leaving group suggests that dissociation is important.
¶ trans effect
certain ligands trans to the leaving group will promote substitution trans to themselves
- Trans effect is the labilising effect a trans ligand has on the leaving group opposite, and rate of substitution of the ligand
Thermodynamic effects ( T is a strong donor )
- T and X donate electron density into the and orbitals of metal centre
- If a -donating trans ligand, and a weaker -donor X, then weaker ligand ( X ) cannot donate electrons to metal as strongly so has a weaker interaction with metal
Kinetic effects ( T is a good π acceptor )
- If T is a good π-acceptor it removes electron density from metal dxy orbital
- Results in nucleophilic attack on the metal being easier
- This lowers the energy of the (5-coord) transition state/intermediate
- Good p-acceptors increase the rate of trans ligand substitution, via stabilisation of 5-coord transition state
¶ Catalysis
The RESTING STATE in catalysis is the intermediate that has the highest concentration.
- It will be the intermediate at the beginning of the section that has the rate-determining step.
¶ Identifying Rate Limiting Step Experimentally
Kinetic Isotope Effect
C-H and C-D bonds are not the same strength
- Zero-point bond energy is related to the reduced mass by
So the energy difference will cause a small difference in rate
- →No difference, this bond is not changed in the RDS
- →This bond breaks in the RDS
- →This bond changes hybridization in the RDS.
¶ Selectivity Determining Step
¶ Overall Catalyst Efficiency
Catalyst efficiency can be measured in a number of ways. Each has limitation
Most common:Activity
- amount of product, per catalyst, per hour.
TurnOver Number (TON)- The number of times a catalyst goes round the cycle.
TurnOver frequency (TOF)- The number of times a catalyst goes round the cycle divided by time catalyst has been on cycle.