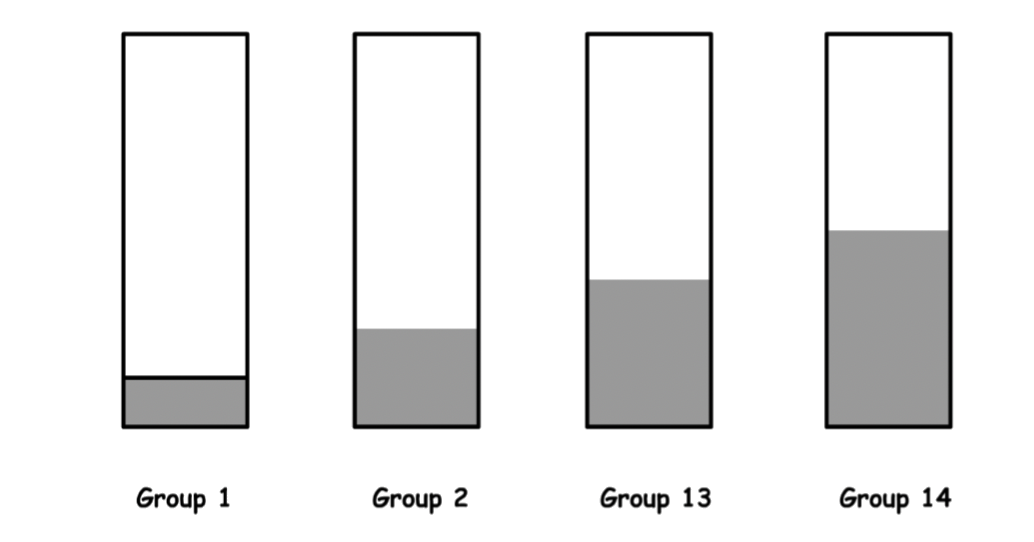¶ Origin of Bands
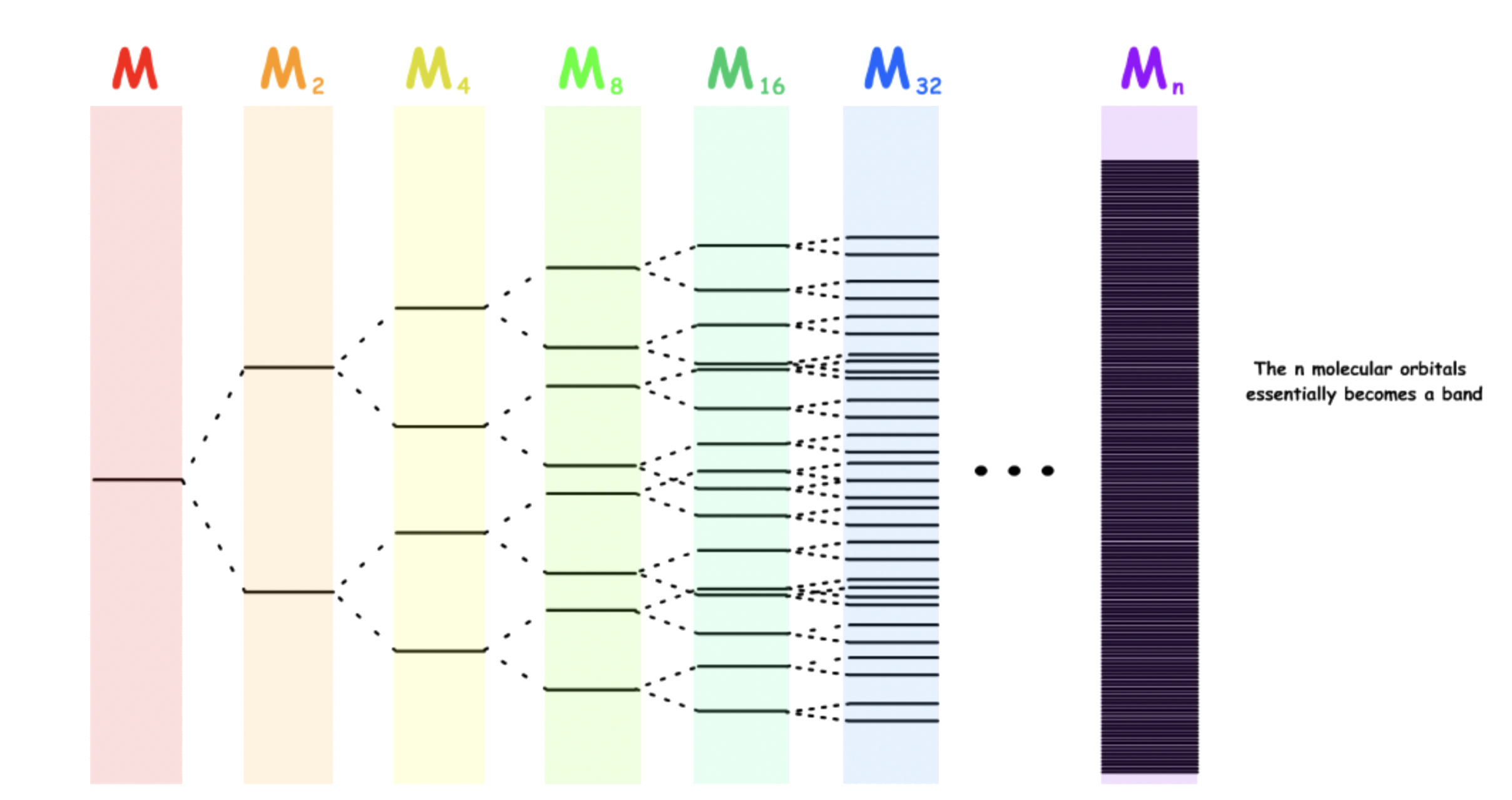
The band theory can be viewed as an extension of the molecular orbital theory for large crystalline solids
- The electrons of a single, isolated atom occupy atomic orbitals, each of which has a discrete energy level
- When two or more atoms join together to form a molecule, their atomic orbitals overlap and form an equal amount of molecular orbitals
For the mixing of any set of orbitals, it will undergo the following process
- As more of the same orbitals are mixed together, the energy difference between adjacent levels decreases
- The orbitals "evolved" from the same orbital will form a band. The energy difference within this band is so small that it is negligible and energy is no longer quantized
- The energy of the adjacent levels is so close together that they can be considered as a continuum, an energy band
- As the number of atoms involved in bonding increases, it will slowly transit from being a gas to a solid, hence, the band theory for solid
- Electrons are allowed to "travel" freely within a band so long as it is no fully occupied
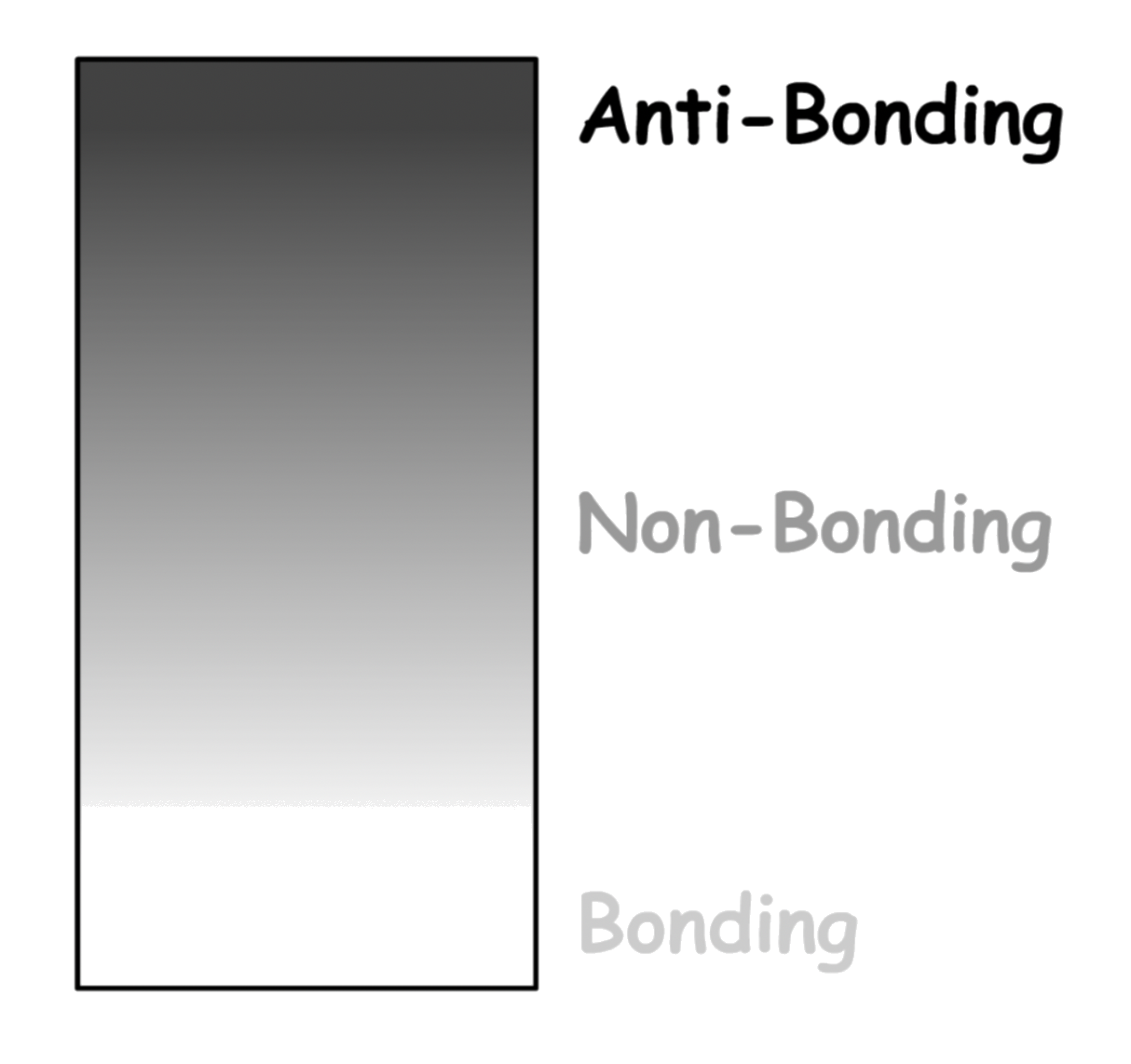
In a typical band, it will transition from bonding character to anti-bonding character
- Not all bands have this trend, some bands may not have non-bonding orbitals, which splits the band into two
¶ Relation between Energy Bands
Since atoms have multiple atomic orbitals, they will also have multiple bands
Each discrete energy level splits into n levels, each with a different energy
- Each set of atomic orbitals will form a band
A band gap is an energy range in a solid where no electron states can exist due to the quantization of energy
- A band gap occurs between the highest member of the band lower in energy and the lowest member of the band higher in energy
The highest member of a band is fully antibonding, while the lowest member of a band is fully bonding
- The energy difference between the bands is smaller than that of their respective atomic orbitals in an isolated atom
¶ Band Width
Orbitals that are deeper in energy cannot overlap with neighboring orbitals properly, so they are only slightly stabilized or destabilized relative to their energies in the isolated atom
- The band is narrow
- Energy remains relatively quantized
The band width is influenced by the extent of overlapping of atomic orbitals and is affected by the following factors
- Coordination number
- The more atomic orbitals participate in the mixing, the stronger the stabilizing and destabilizing interaction
- The higher the coordination, the wider the band
- Orbital size
- The larger the atomic orbital, the poorer the overlapping
- Atomic orbitals that are large and diffused forms narrow band
- Orientation
- Head-on overlapping is more effective than side-to-side overlapping
- Energy separation
- The interaction between orbitals increases as the difference in similarity in energy levels decrease
- Affects compounds
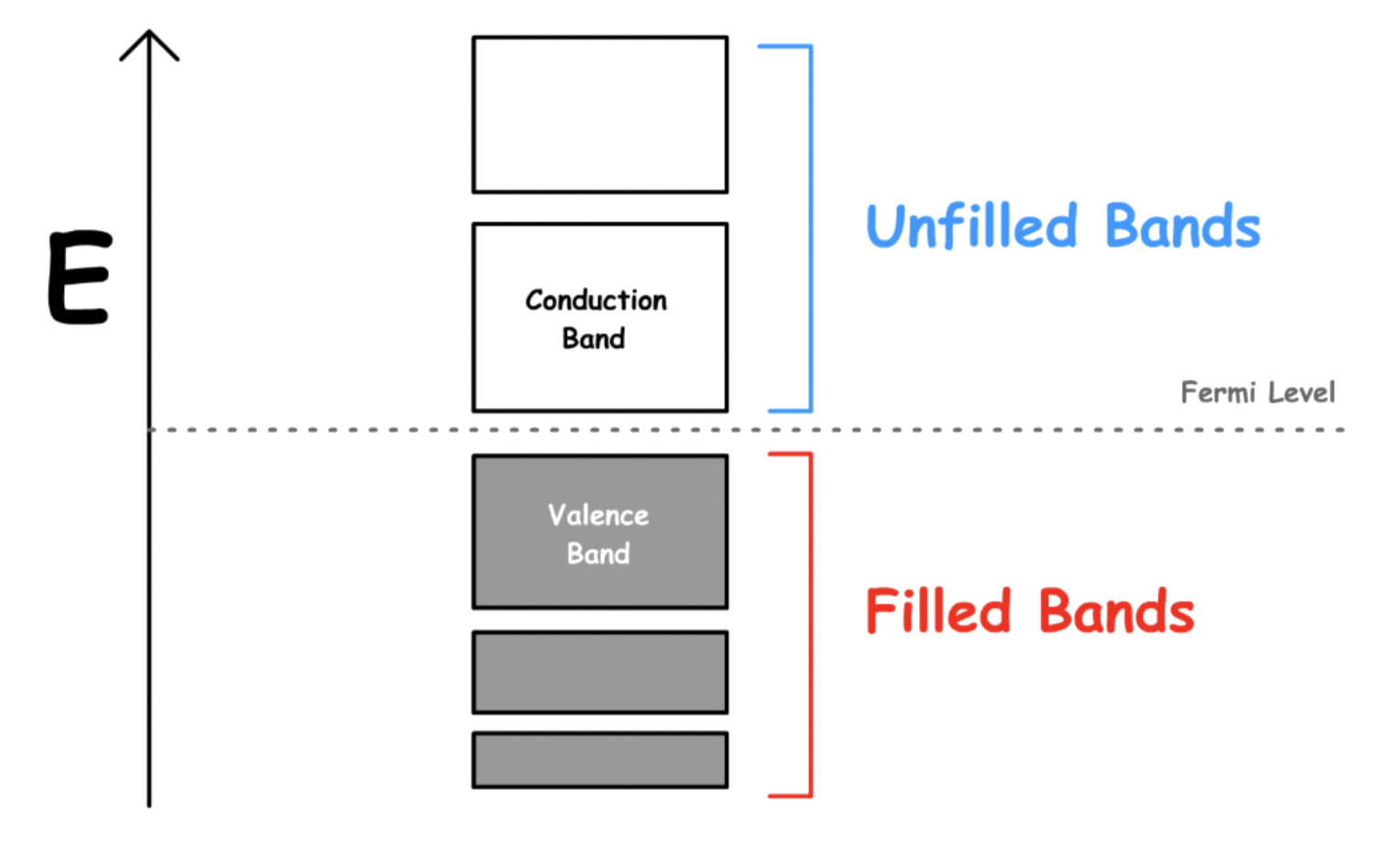
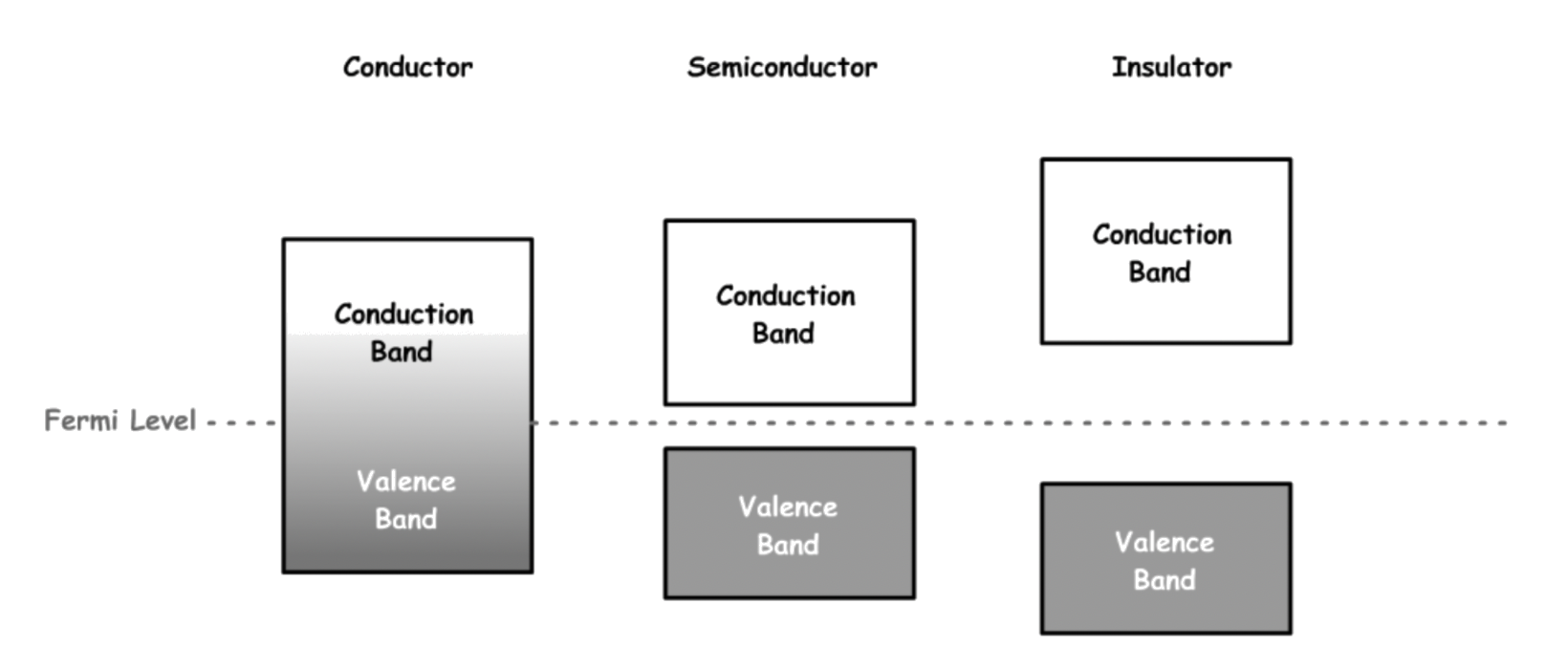
¶ Valence and Conduction bands
The Fermi level is the highest energy level that is populated at 0 K
- The Fermi level does not necessarily correspond to an actual energy level (in an insulator the Fermi level lies in the band gap), nor does it require the existence of a band structure
- The valence band and conduction band are the bands closest to the Fermi level
- On a graph of the electronic band structure of a material, the valence band is located below the Fermi level, while the conduction band is located above it
- The valence band is the highest range of energies in which electrons are normally present at absolute zero temperature
¶ Electrical conductivity
The band gap between the valence and conduction bands is crucial to the electrical conductivity of a material
In solids, the ability of electrons to act as charge carriers depends on the availability of vacant electronic states
- The vacant levels allow the electrons to increase their energy (i.e. accelerate) when an electric field is applied
- When electrons are in a band that is completely occupied, they cannot transit to another energy state, which means they cannot carry charges
- When electrons are in a partially filled band, transition is possible, therefore charges can be carried
In order to conduct electricity, electrons must be in a partially filled band, which can be done by exciting electrons from the valence band to the conduction band
- It is also possible to partially fill the conduction band or partially remove electrons from the valence band by forming compounds
Hence, the electrical conductivity depends on the band gap between the valence band and the conduction band
For conductors, the conduction bands and valence bands overlap to form one band
- The merged band is partially filled, so it can conduct electricity
The band gap of the conduction band and the valence band of a semiconductor is small
- At 0 K, the conduction band is not populated, so it is unable to conduct electricity
- As the temperature increases, the thermal energy excites the electrons in the valence band to the conduction band, causing the conduction band and the valence band to be partially filled
The band gap of the conduction band and the valence band of a semiconductor is large
- The energy required to excite electrons into the conduction band is so large that it may melt the material or break bonds
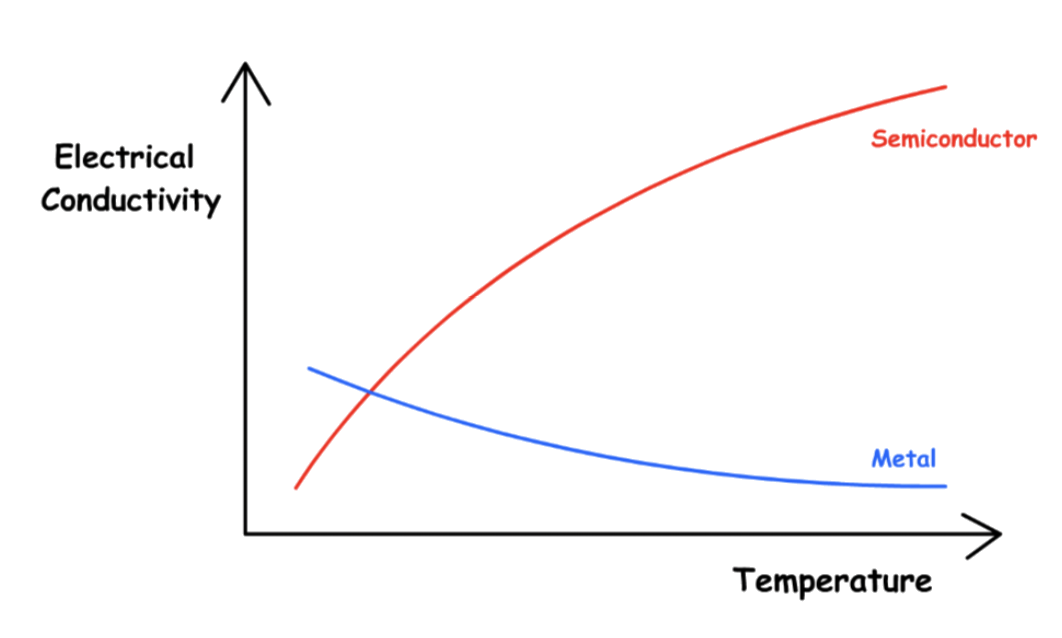
¶ Temperature and Conductivity
The electrical conductivity of semiconductors increases rapidly with temperature
- Thermal energy can excite electrons across the band gap in a semiconductor, increasing the temperature increases the number of electrons that have sufficient kinetic energy to be promoted into the conduction band
The electrical conductivity of metal decreases steadily as the temperature increases
- In a metal, as an electron travels through the crystal in response to an applied electrical potential, it cannot travel very far before it encounters and collides with a metal nucleus
- The more often such encounters occur, the slower the net motion of the electron through the crystal, and the lower the conductivity
- As the temperature of the solid increases, the metal atoms in the lattice acquire more and more kinetic energy. Because their positions are fixed in the lattice, however, the increased kinetic energy increases only the extent to which the nuclei vibrate about their fixed positions
- At higher temperatures, therefore, the metal nuclei collide with the mobile electrons more frequently and with greater energy, thus decreasing the conductivity
¶ Materials
¶ Metal
Main Group Metals
Simple metals
- High-coordinate structures
- Close packing
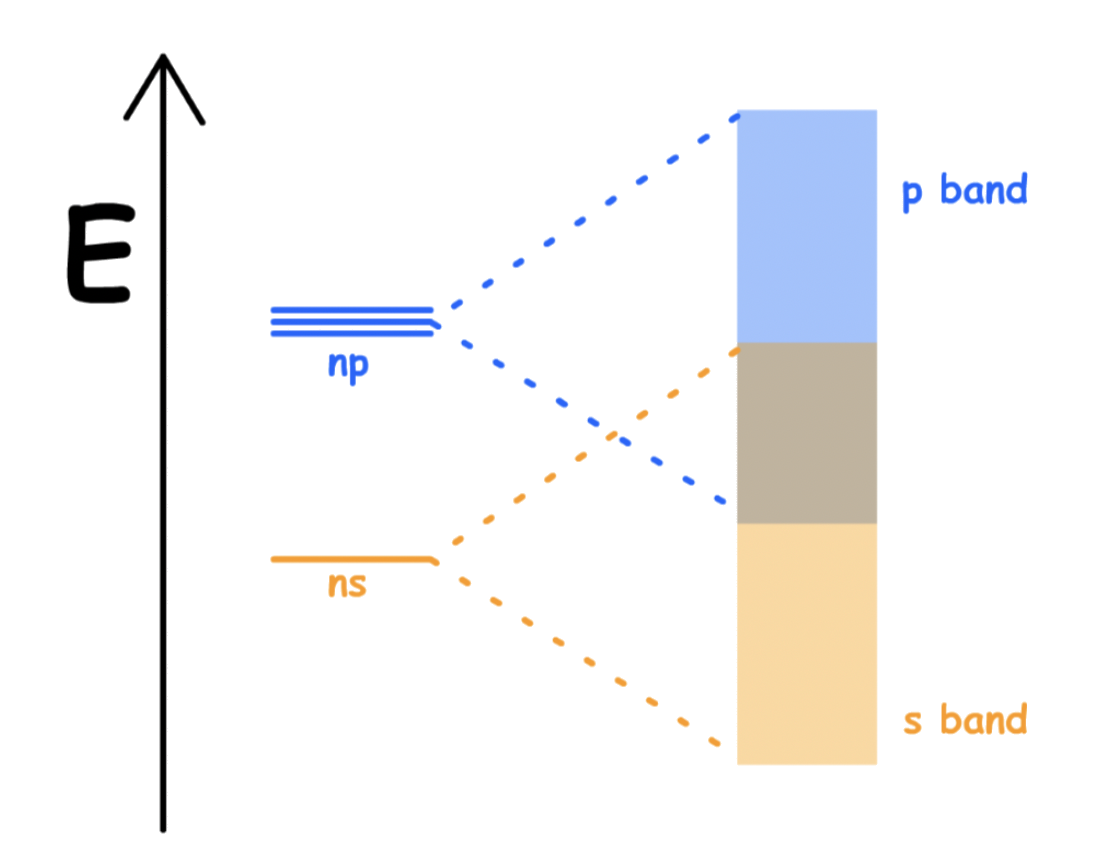
The energy gap between the atomic s and p valence orbitals which forms the MOs are small
- The s and p valence band overlaps and merges to form a single band
- A single s-p band ( no band gap )
The s-p band has a total of 4N orbitals, so it can house up to 8N electrons
- N is the total number of atoms combined
The occupancy of the band depends on the number of valence electrons of the atoms
- For group 1 metals, one eighth of the band is filled
- For group 2 metals, a quarter of the band is filled
- For group 13 metals, three eighth of the band is filled
- For group 14 metals, three eighth of the band is filled
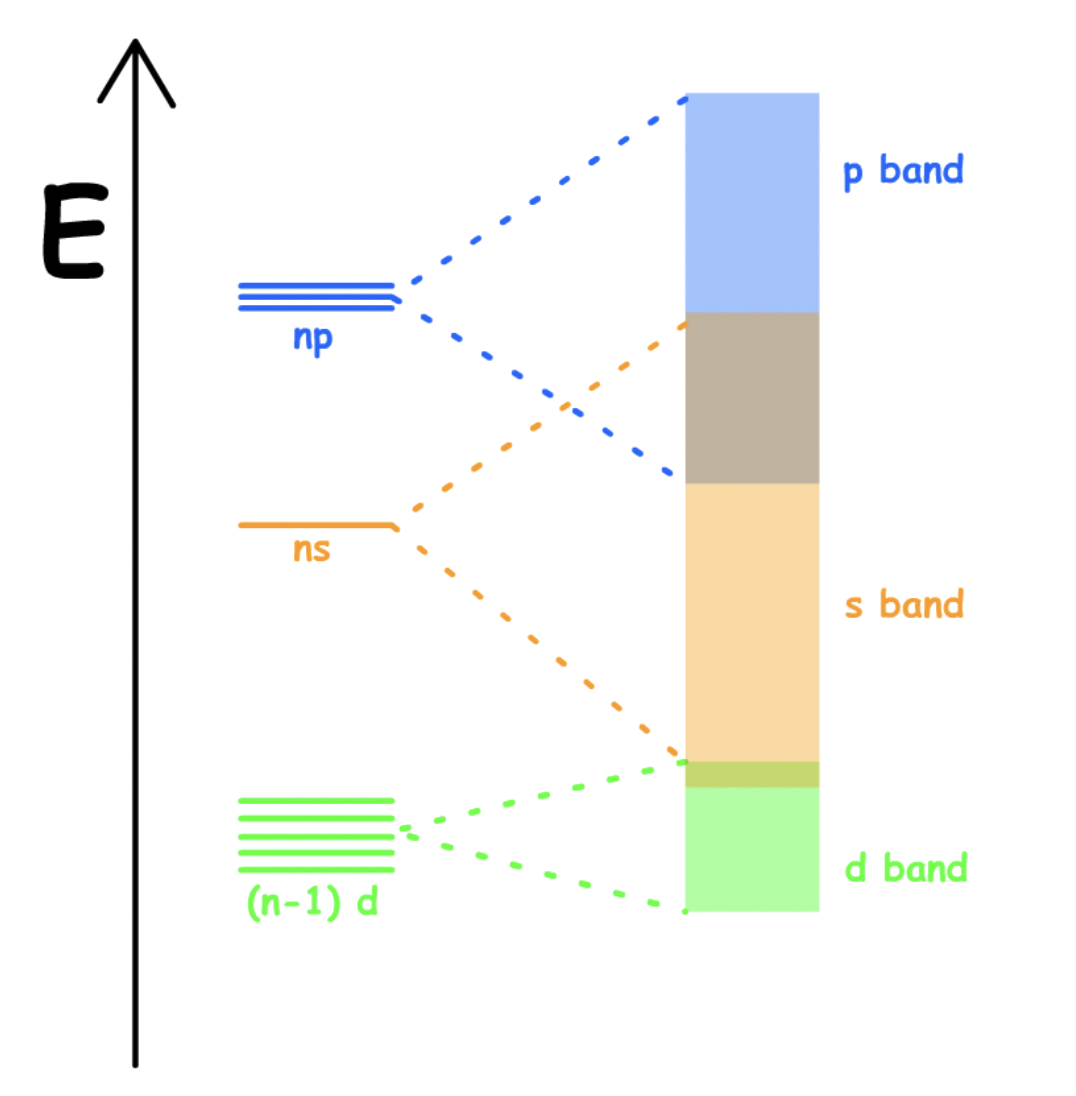
Transition Metals
High-coordinate structures
- Close packing
The energy gap between the atomic s and p valence orbitals which forms the MOs are small
- The s, p and d valence band overlaps and merges to form a single band
- A single s-p-d band ( no band gap )
Since the s-p-d bands are partially filled, transition metals can conduct electricity
The valence electrons of transition metals occupy either their valence ns, (n − 1)d, and np orbitals (with a total capacity of 18 electrons per metal atom) or their ns and (n − 1)d orbitals (a total capacity of 12 electrons per metal atom)Metals with 6 to 9 valence electrons (which correspond to groups 6–9) are those most likely to fill the valence bands approximately halfway
- Those electrons therefore occupy the highest possible number of bonding levels, while the number of antibonding levels occupied is minimal
- Not coincidentally, the elements of these groups exhibit physical properties consistent with the presence of the strongest metallic bonding, such as very high melting points