¶ Nucleophilic Aliphatic Substitution
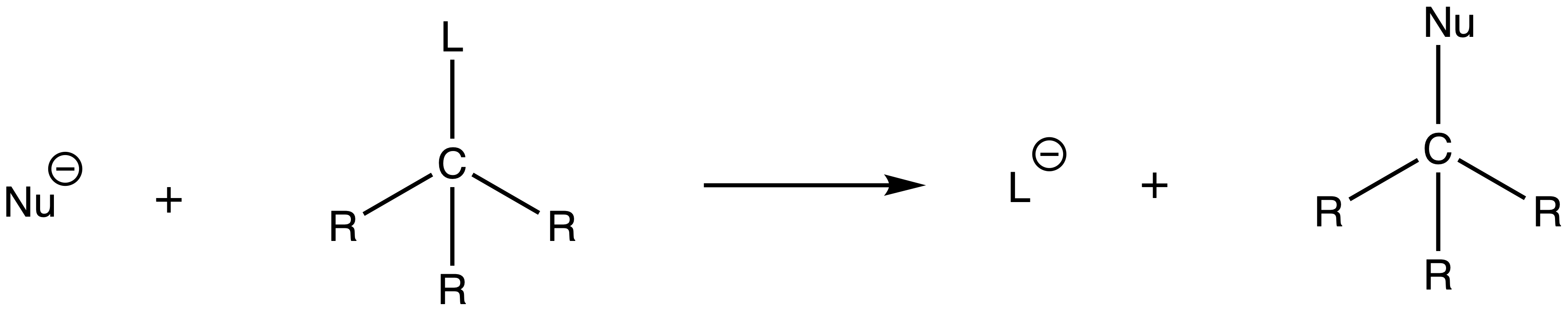
There are two types of nucleophilic substitution sp3 carbons undergo: SN1 and SN2
- Both reactions involve the attack of a nucleophile and the ejection of a leaving group
The numbering system of the two nucleophilic substitution originates from their rate equation
- The rate equation of SN1 is first order and only depends on the concentration of the electrophile ( The parent molecule )
- The rate equation of SN2 is second order and depends on the concentration of the electrophile ( The parent molecule ) and the nucleophile
¶ Mechanism
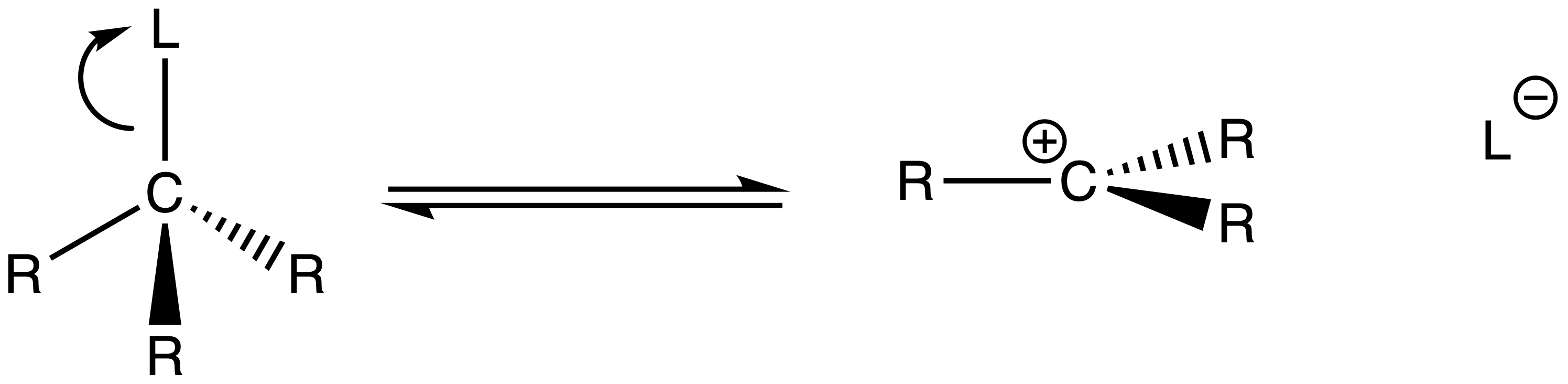
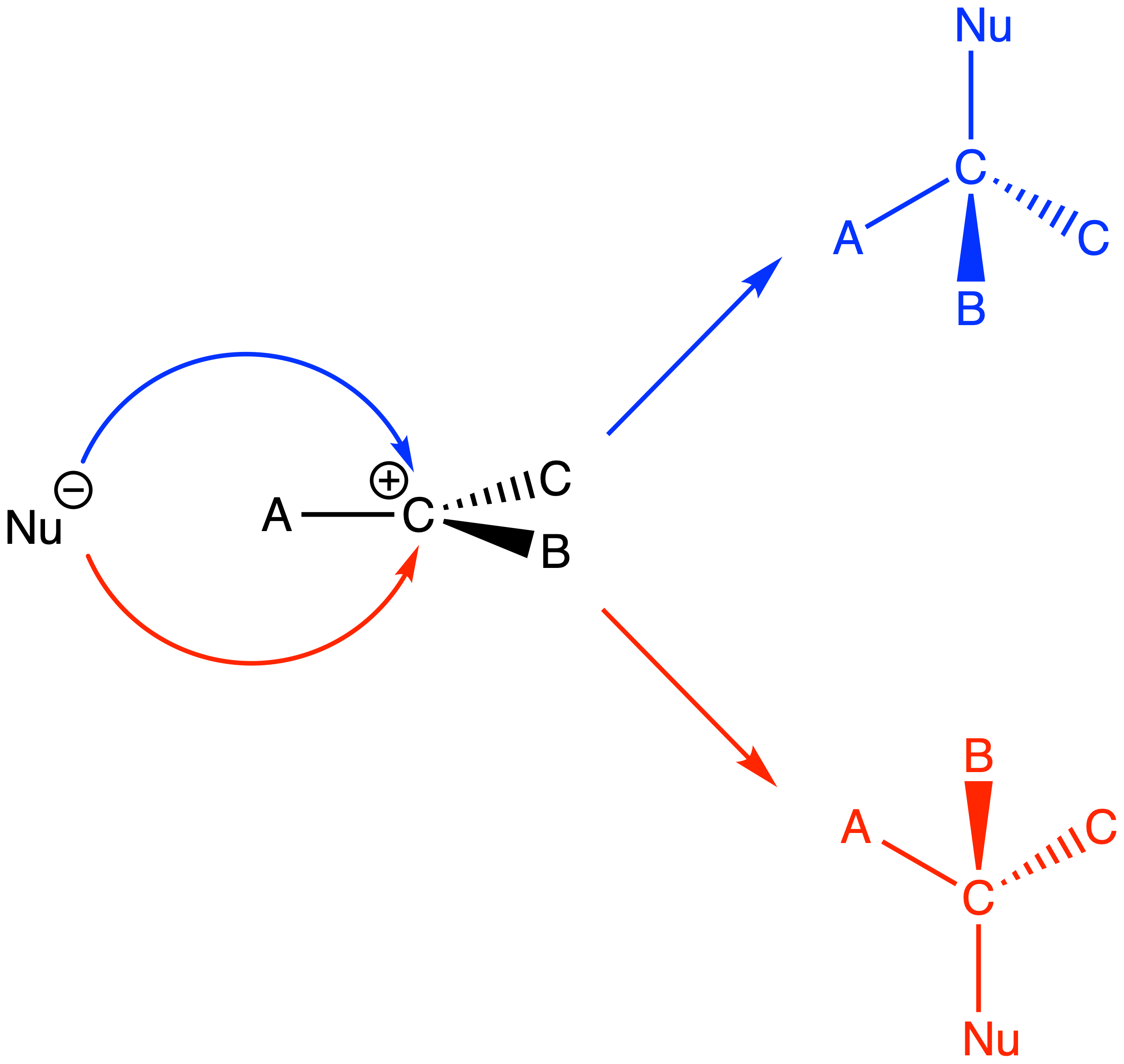
The mechanism of SN1 reactions
- The bond between carbon and the leaving group gets longer and longer until the bond is dissociated
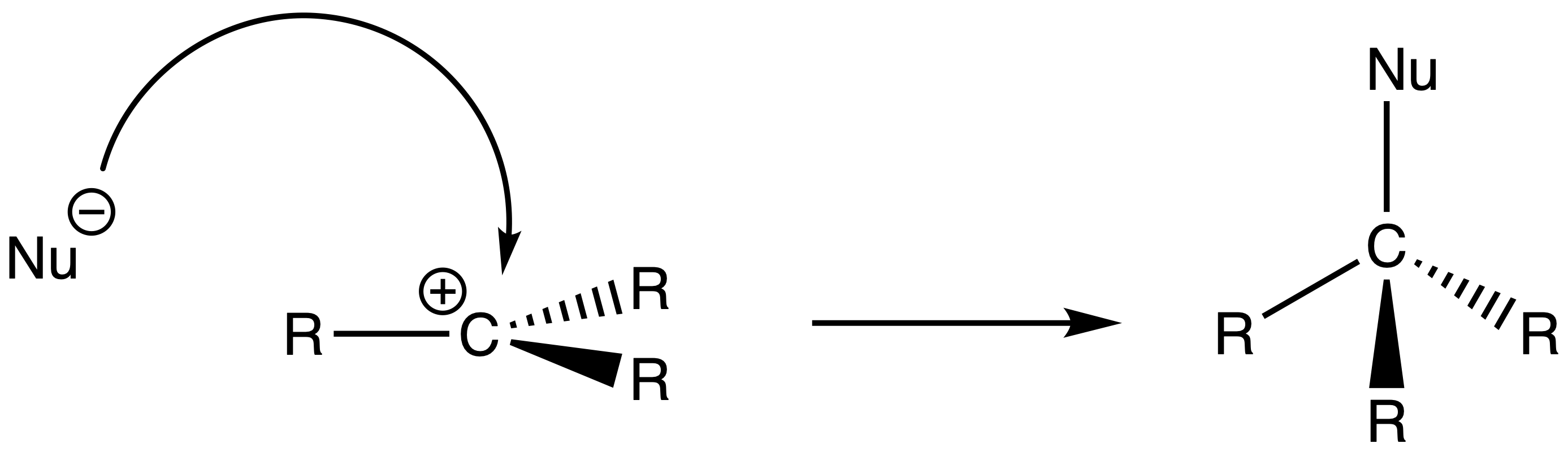
- The nucleophile attacks the carbocation to form the product
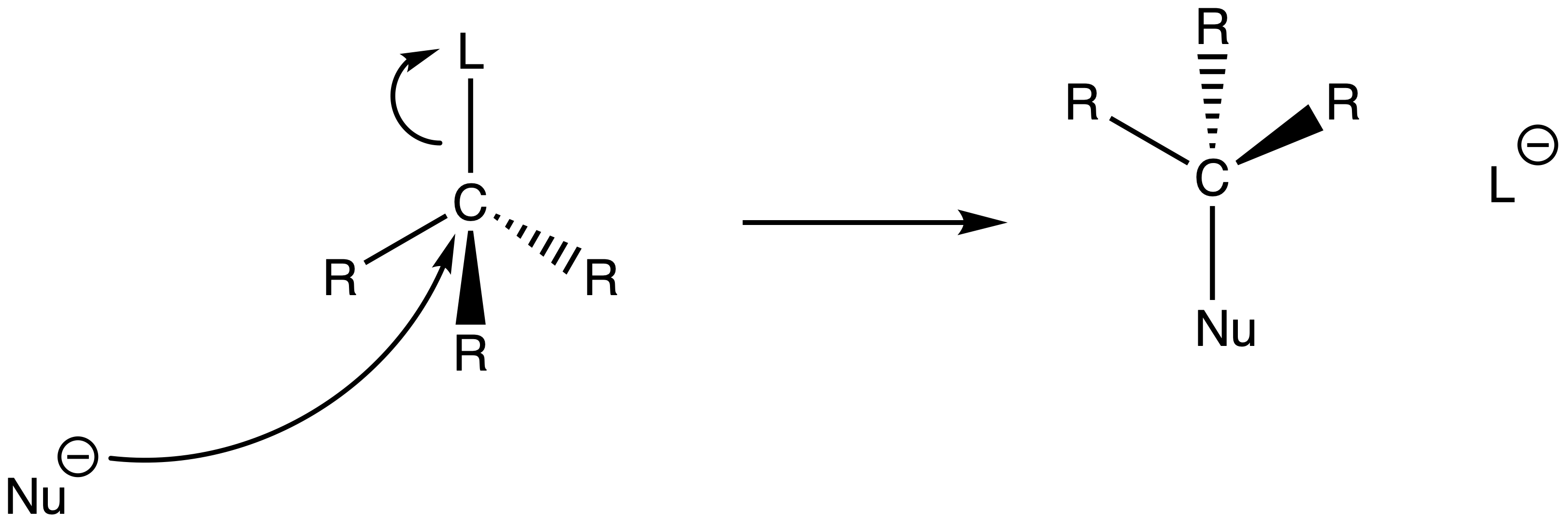
The mechanism of SN2 reactions
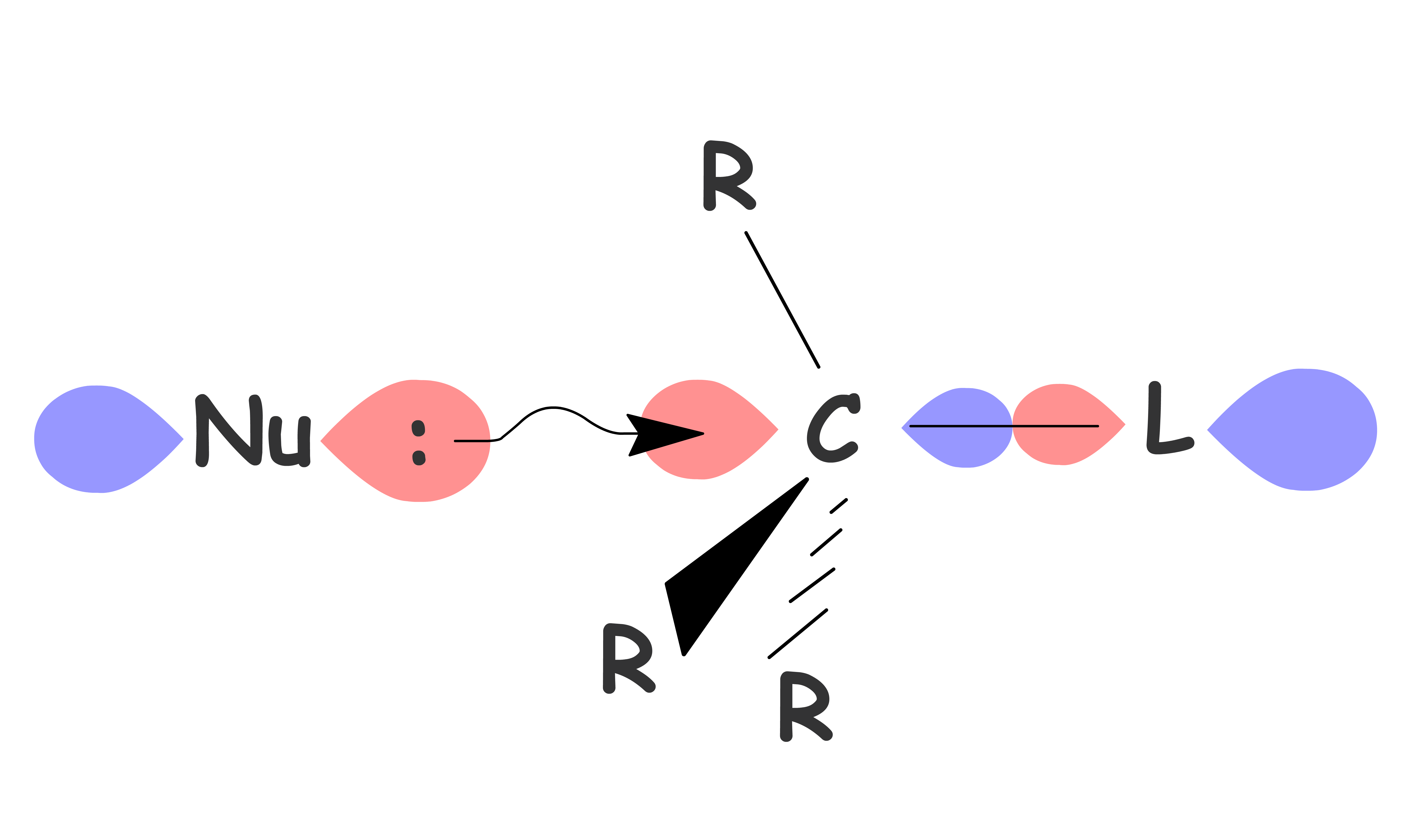
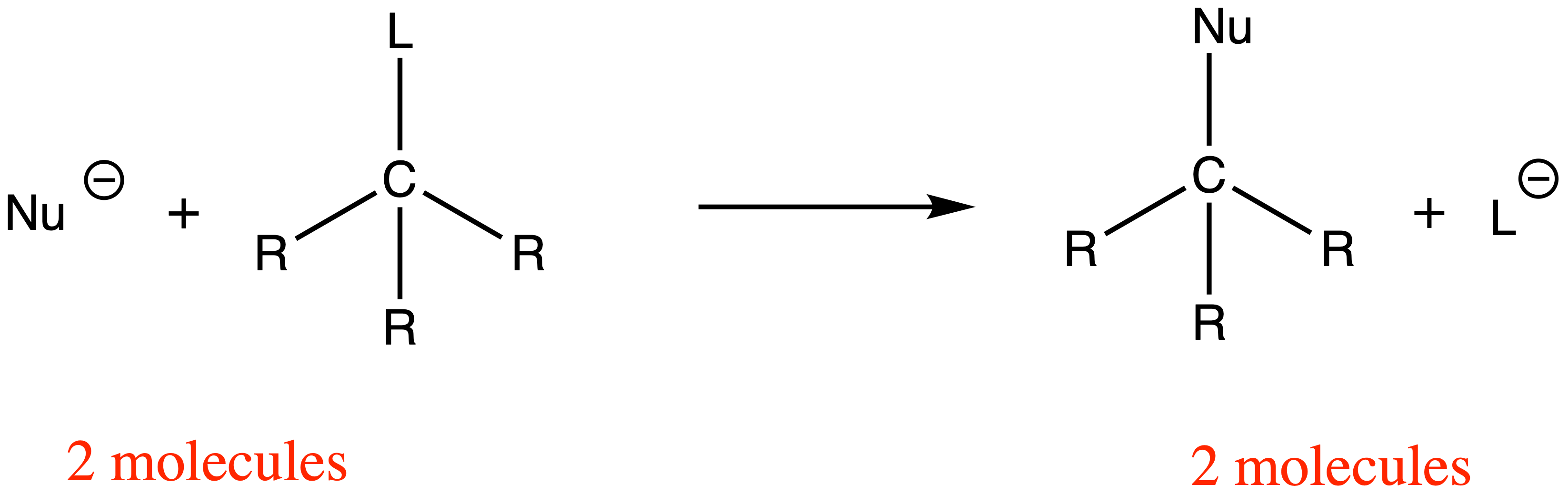
- A nucleophile attacks the sp3 carbon and eject the leaving group at the same time through a concerted mechanism
¶ Stereoselectivity
SN1
For SN1 reactions, the product will be a racemic mixture ( 50% of each enantiomer )
- This is because the nucleophile can attack either end of the prochiral center of the carbocation
Sometimes, the mixture of products may not be cleanly divided into two 50% portions
- This happens when the leaving group is still electrostatically attracted to the carbocation, and may linger in the neighborhood, which hinders the approach of the nucleophile from that side, leading to a slight preference for inversion
- This is especially true for poor leaving groups and less polar or aprotic solvents
SN2
For SN2 reactions, the chirality at the reacting site will be inverted as the nucleophile can only attack the carbon directly behind the C–L bond
- The HOMO of the nucleophile needs to overlap with the σ* orbital of the C–L bond
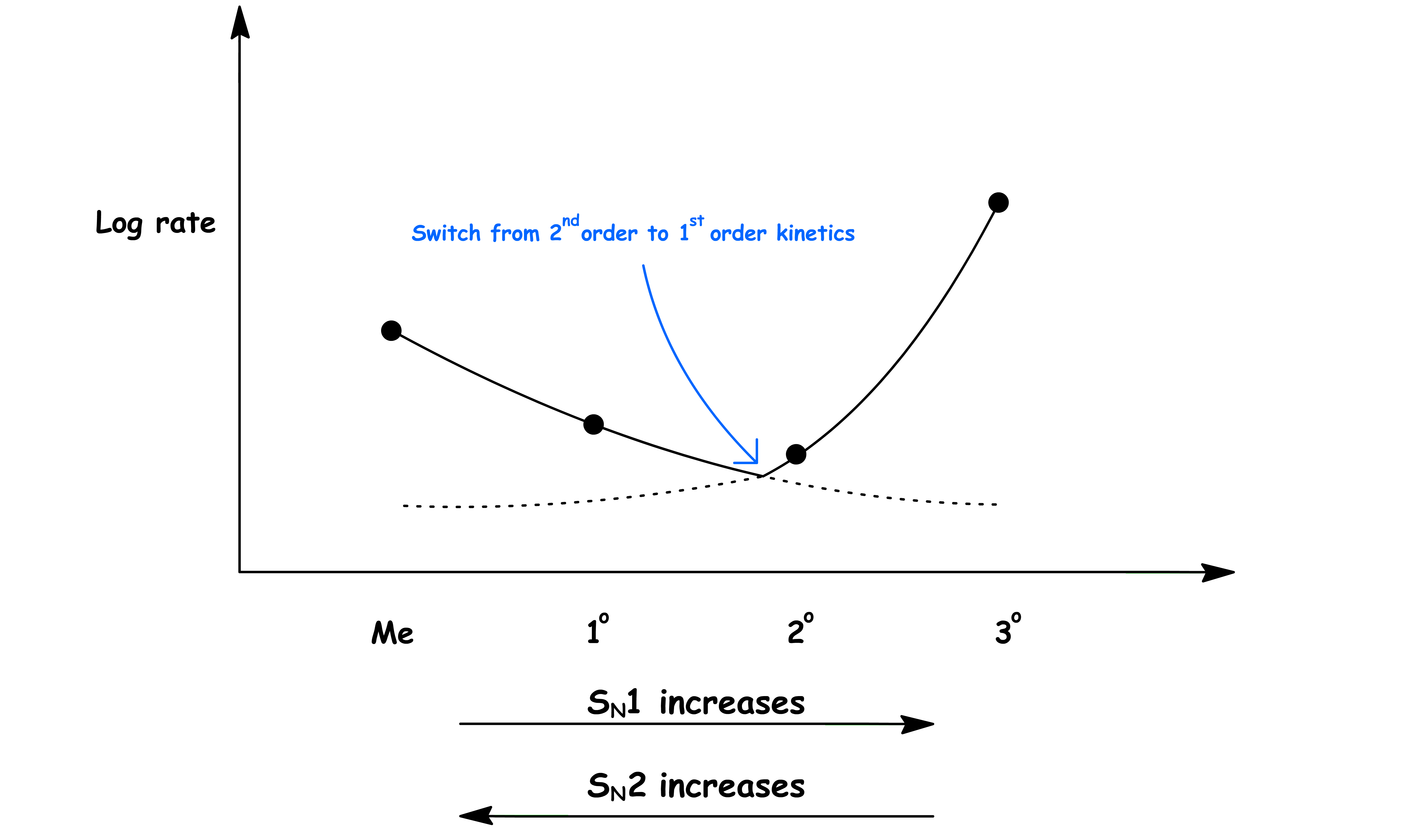
¶ Preference between SN1 and SN2 due to the structure of the Electrophile
SN1 reaction involves a carbocation intermediate. According to the Hammond's Postulate, anything that can stabilize the carbocation can stabilize the transition state and thus increase the rate of reaction
Carbocations with a higher degree of substitution will be stabilized to a greater degree and SN1 reaction is favored
The formation of the carbocation is the rate-determining step
SN2 reaction is sensitive to steric hinderance as the nucleophile must attack directly behind the C–L bond to maximize the orbital overlap
- If there is steric crowding around the reacting site, the nucleophile will not be able to access the carbon and the rate of reaction will drop
- Even primary leaving groups can have serious steric crowding as nearby bulky groups can branch out and prevent the nucleophile from attacking
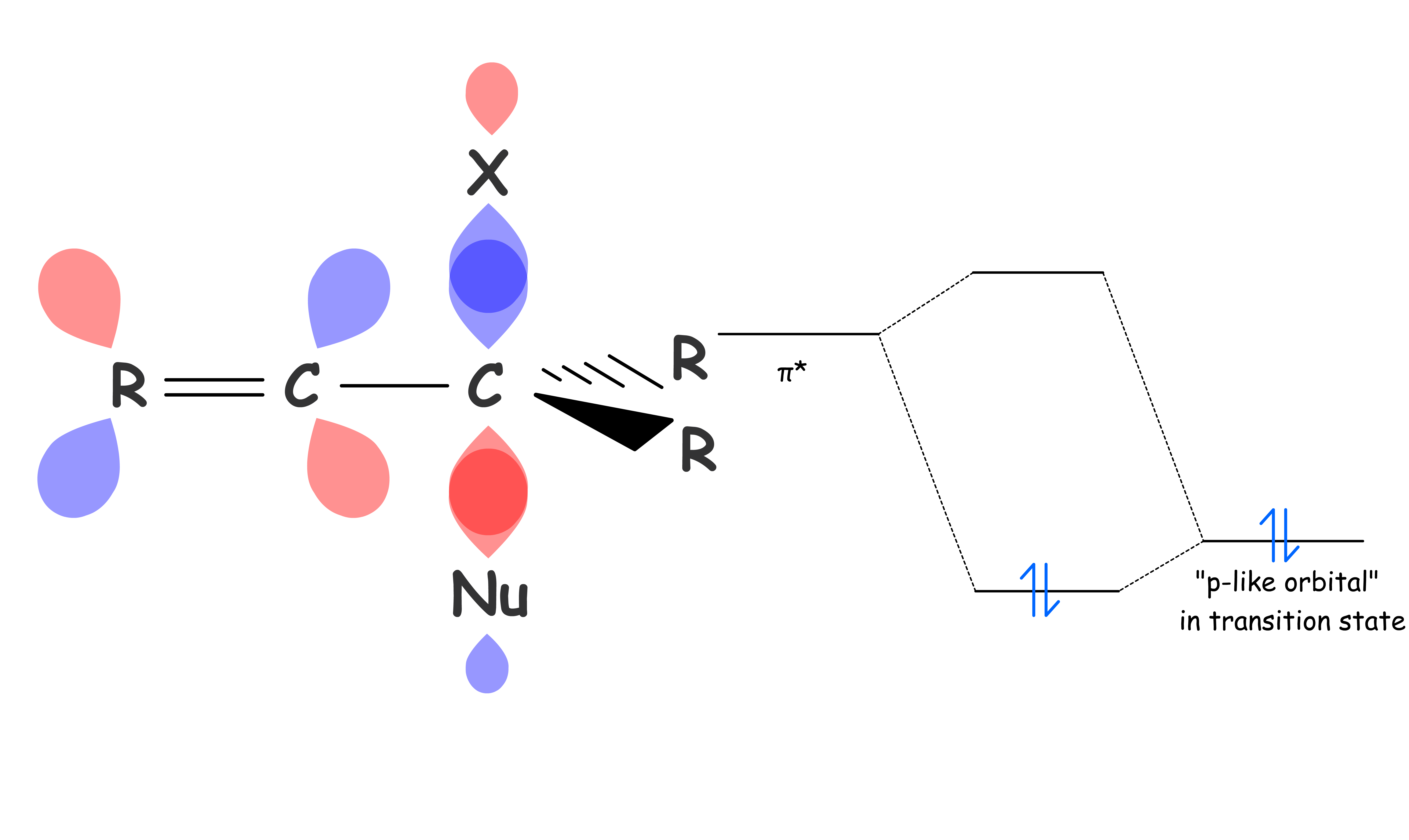
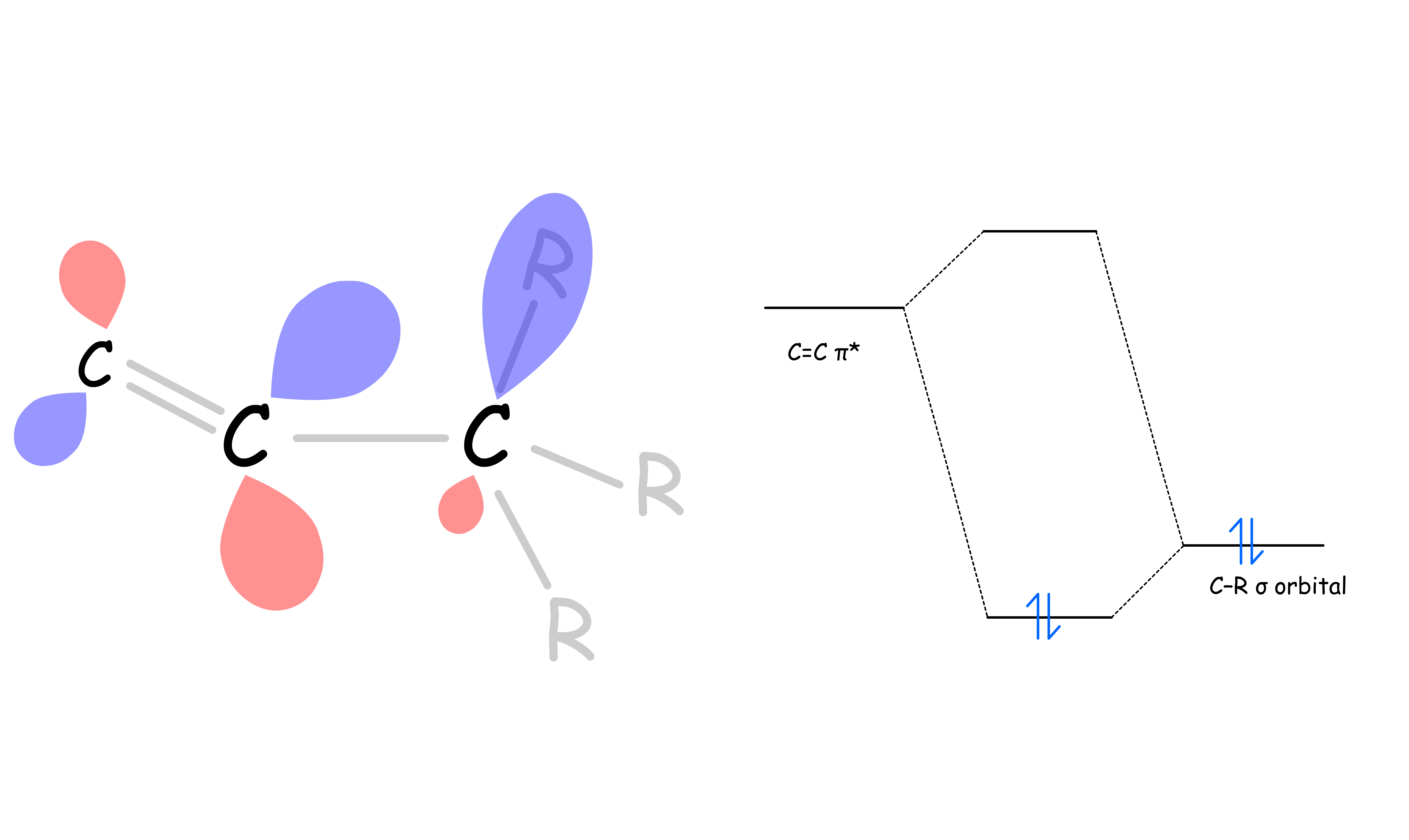
SN2 reaction can be stabilized by nearby double bonds through pi conjugations
- The carbon in the transition state has a p-like filled orbital, it can be stabilize by an empty π* orbital as they are close in energy
- The stabilization effect is enhanced if the carbon is double bonded to electronegative atoms as they can pull the π* lower in energy which allows for better overlapping
The pathway of nucleophilic substitution reaction will change as the substitution of the sp3 carbon changes
- 2° leaving groups may undergo the other pathway depending on the reacting condition
¶ Preference between SN1 and SN2 due to the Nucleophile
In SN1 reactions, nucleophiles are unable to affect the rate of reaction, meaning that the identity and the concentration of the nucleophile have no effect on the rate of reaction
- This is because carbocation is highly unstable, so it will react with strong and weak nucleophiles equally fast
For SN2 reactions, the nucleophiles are involved in the rate-determining step, so the concentration and the type of nucleophile used will affect the rate of reaction
- There are two factors that affect the rate of SN2 reactions
1. pKaH of the nucleophile
- For nucleophiles that are based on the same heteroatom, the rate of reaction increases for higher pKaH values
- pKaH alone is often inaccurate for other situations as it only reflects the philicity of the nucleophile towards hydrogen ions
2. Hardness and Softness of the nucleophile
- The HOMO of soft nucleophile is higher than that of hard electrophile
- Hence, the gap between the LUMO of the electrophile and the HOMO of the nucleophile is smaller, allowing more effective overlaps
The type of nucleophile and the concentration of nucleophile can influence whether the reaction proceeds via SN1 or SN2
- SN2 reactions are controlled by HOMO–LUMO interactions, so soft nucleophiles, which have high energy HOMO, will prefer to react in a SN2 manner
- The rate of SN2 reactions increase with the concentration of the nucleophile, so a high concentration of nucleophile will favor the SN2 reaction over the SN1 reaction
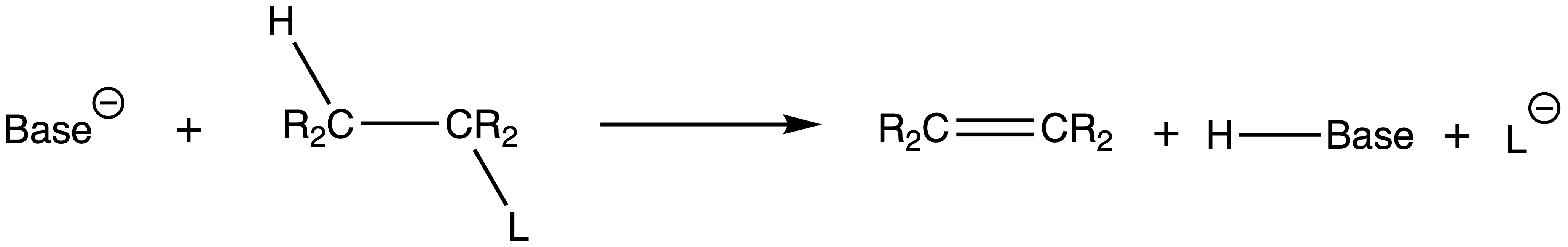
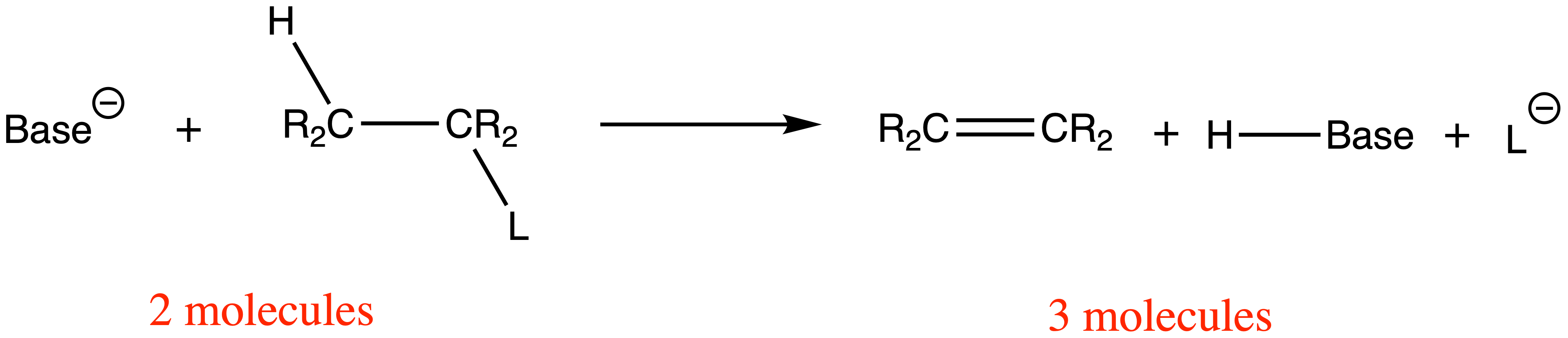
¶ Elimination Reactions
There are two types of eliminations sp3 carbons undergo: E1 and E2
- Both reactions involve a base picking up a β-hydrogen and the ejection of the leaving group and the formation of a double bond
The numbering system of the two elimination reaction originates from their rate equation
- The rate equation of E1 is first order and only depends on the concentration of the Lewis acid ( The parent molecule )
- The rate equation of E2 is second order and depends on the concentration of the Lewis acid ( The parent molecule ) and the base
¶ Mechanism
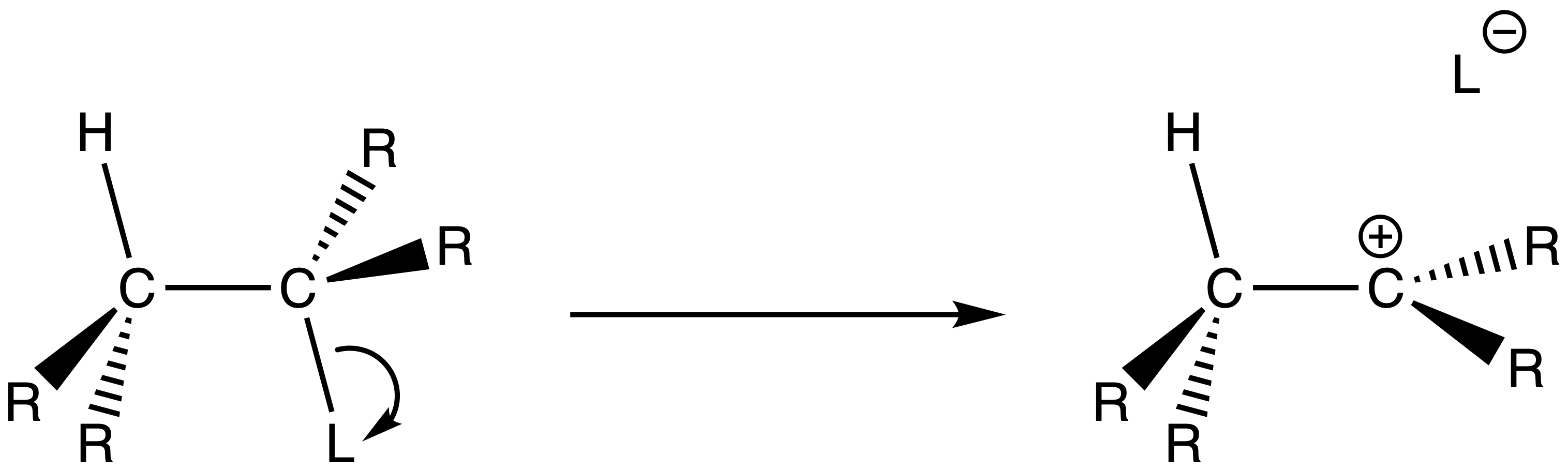
The mechanism of E1 reactions
The bond between carbon and the leaving group gets longer and longer until the bond is dissociated
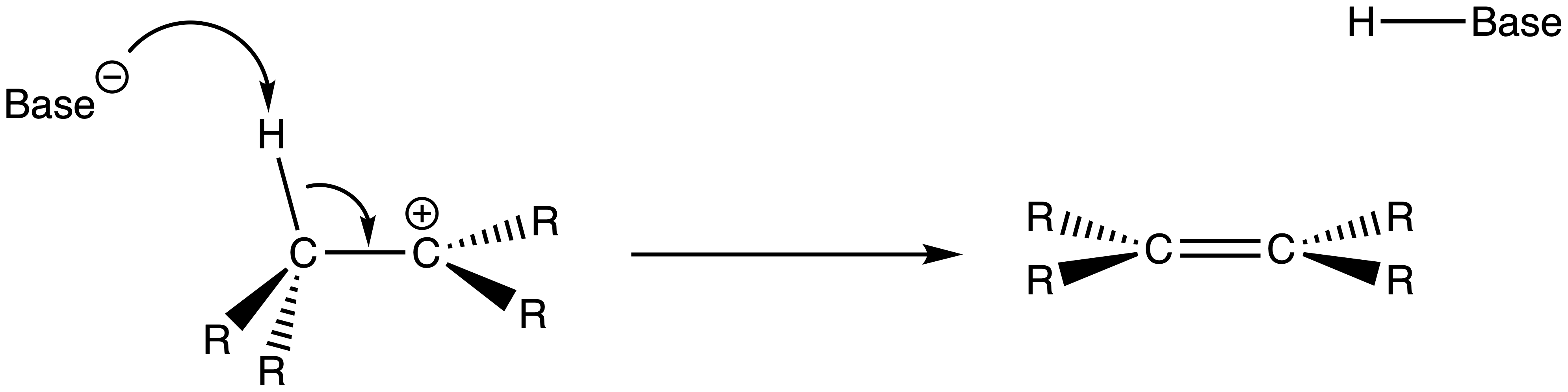
A base comes along and picks up the β-hydrogen and a carbon-carbon double bond is formed
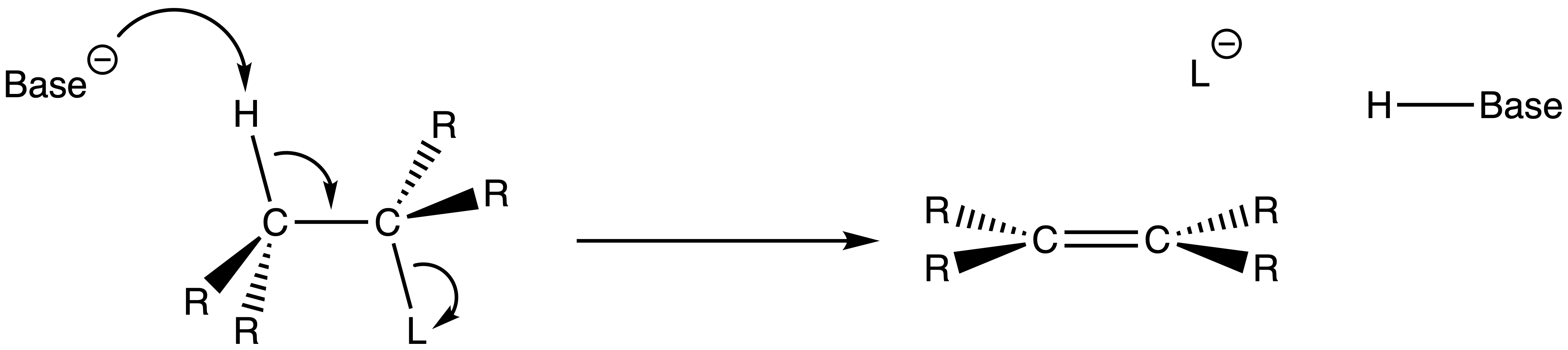
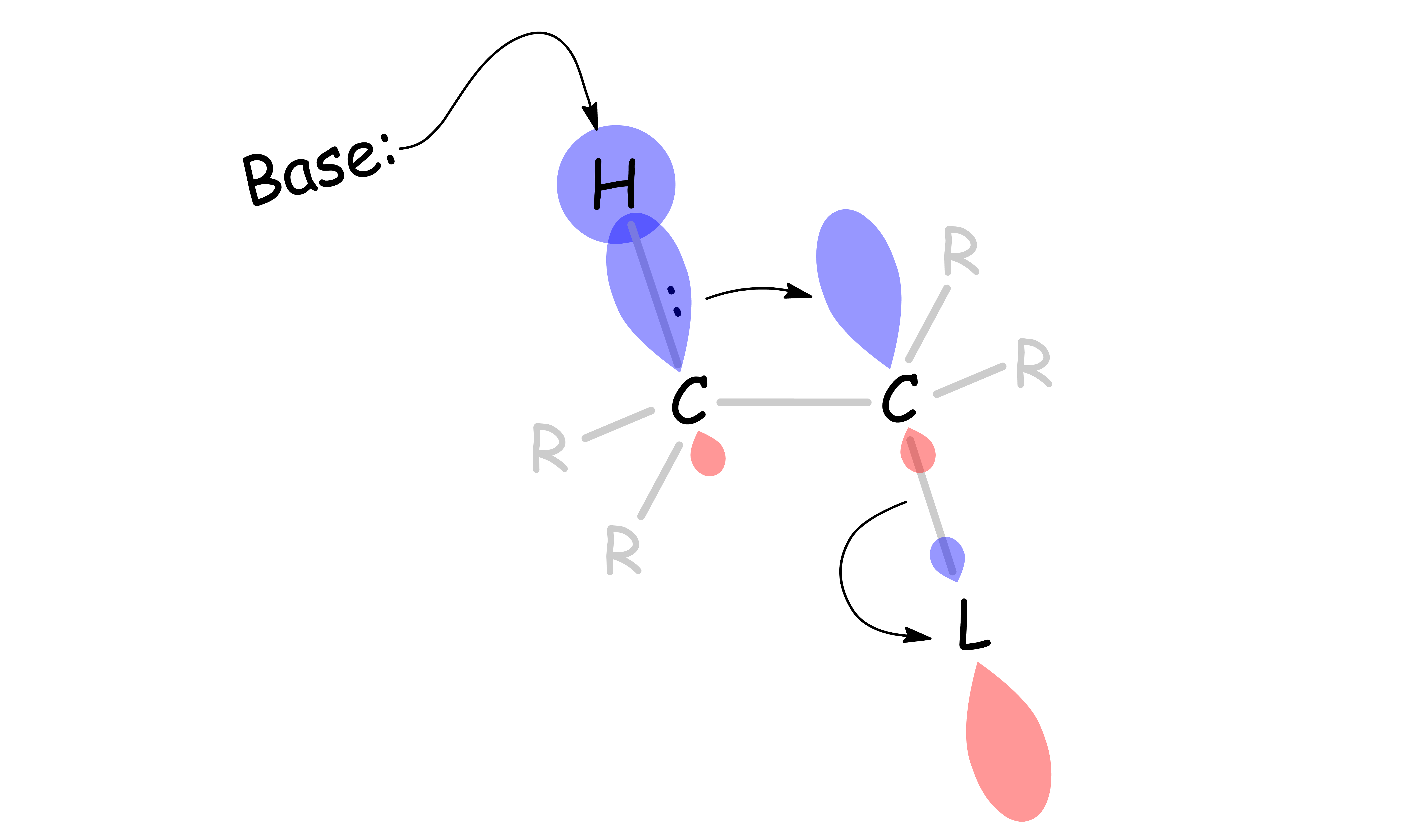
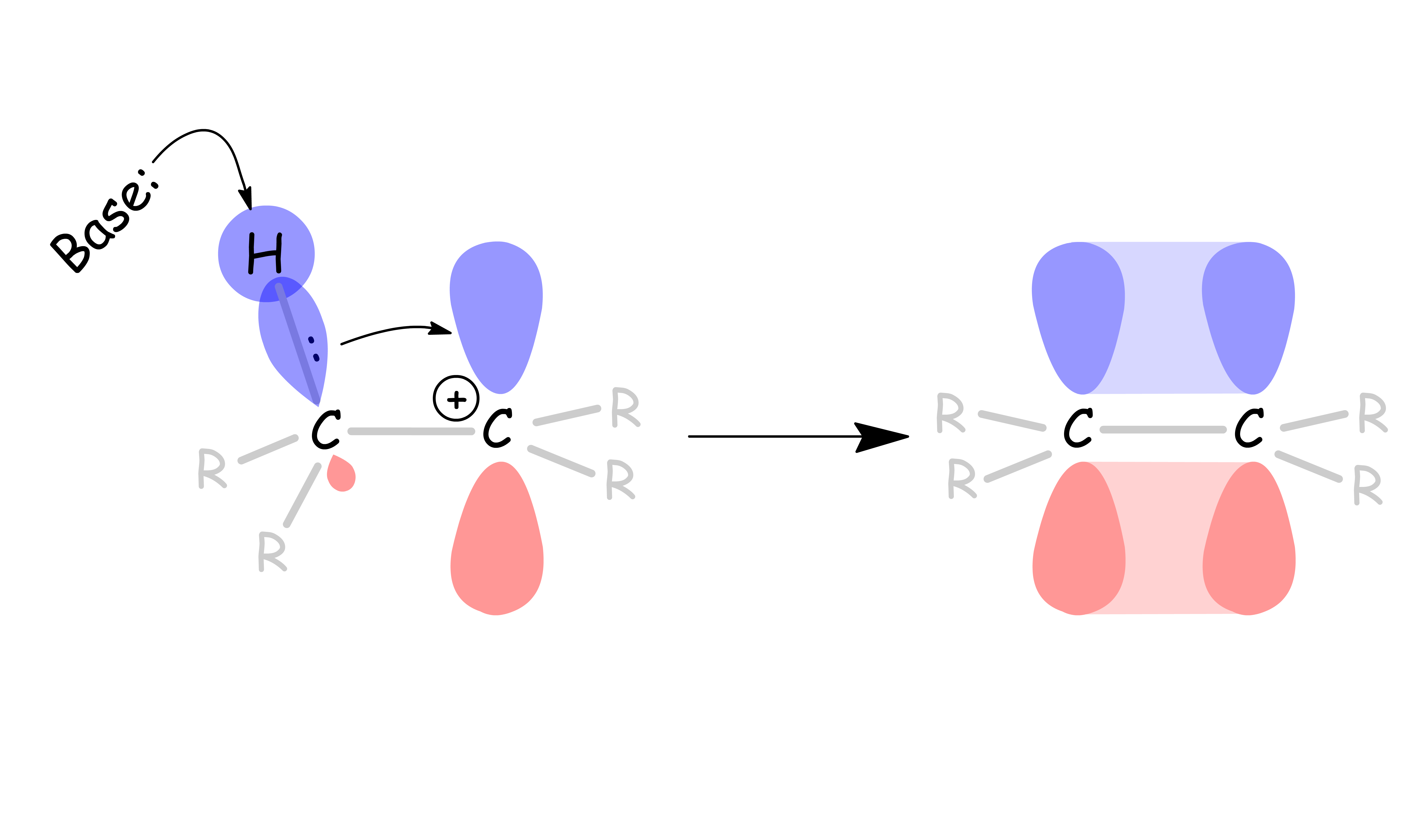
The mechanism of E2 reactions
- A base attacks the β-hydrogen and ejects the leaving group at the same time through a concerted mechanism
¶ Regioselectivity
Both E1 and E2 reactions obey the Saytzev Rule when the base is not sterically hindered
- Saytzev Rule implies that base-induced eliminations will lead predominantly to the alkene in which the double bond is more highly substituted
- Highly substituted alkenes are more stable due to sigma-conjugation
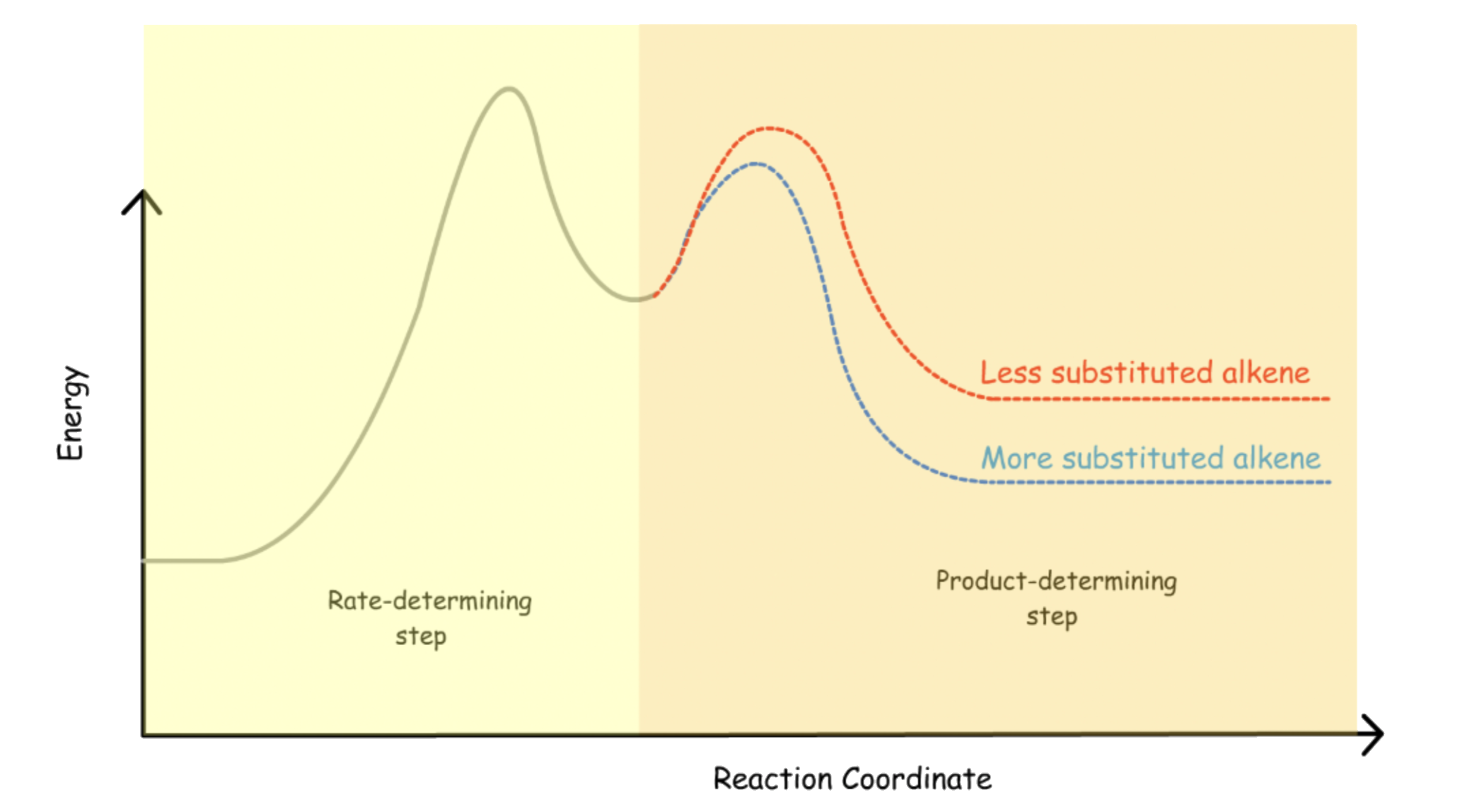
For E1 reactions, anything that stabilizes the alkene will stabilize the transition state of the product determining step ( 2nd step )
For E2 reactions, anything that stabilizes the alkene will stabilize the transition state of the rate-determining step
When the base is sterically hindered ( The base is bulky ), both E1 and E2 reactions obey the Hofmann's Rule
- Hofmann's Rule states that steric effects have the greatest influence on the outcome of the Hofmann or similar elimination reaction
- The bulky base will pick up the least hindered proton and so the major product will be the least substituted alkene
¶ Stereoselectivity
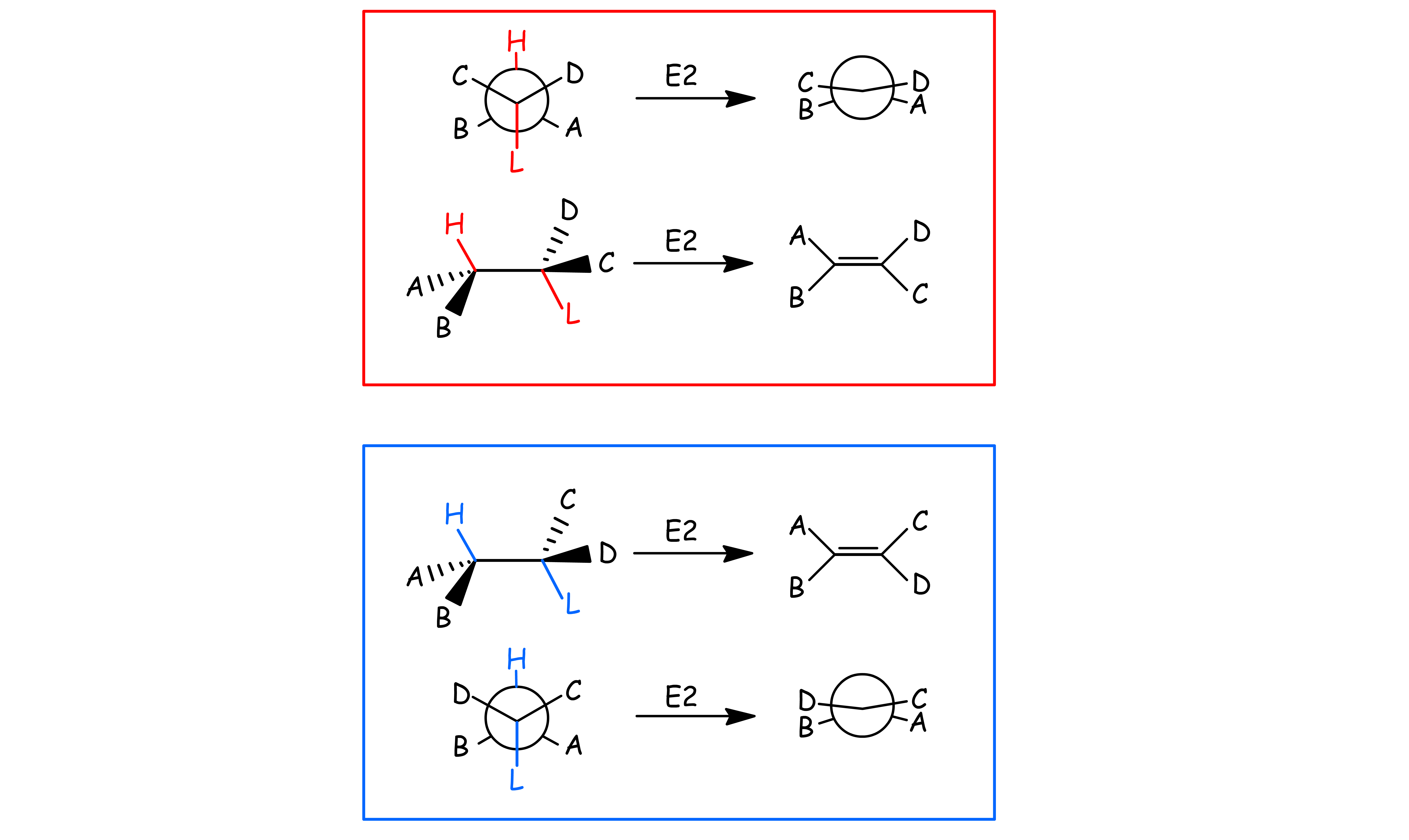
E2 reactions occur most rapidly when the H-C bond and C-Leaving Group bonds involved are co-planar, most often at 180o with respect to each other
- The β-hydrogen is anti-periplanar with respect to the leaving group
- This conformation allows the C – H σ orbital to overlap and donate electron density efficiently to the C – R σ* orbital:
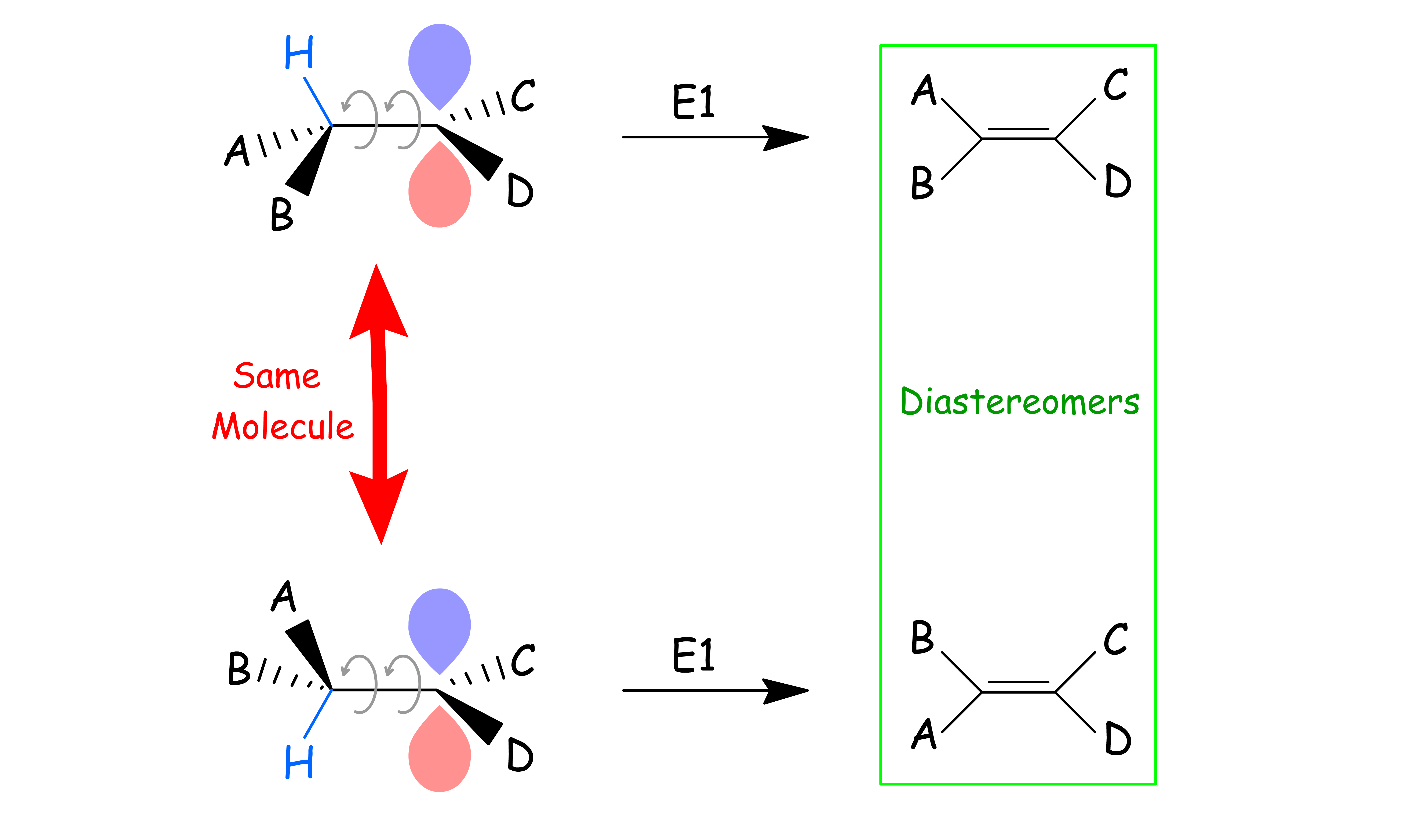
Unlike E2 reactions, E1 reactions are not stereospecific
- Thus, the hydrogen is not required to be anti-coplanar to the leaving group because the leaving group is gone
- However, the C – H σ orbital needs to align with the empty p-orbital of the carbocation such that it can donate electron density to it
Since E2 reactions are stereospecific, stereoisomeric starting materials afford stereoisomerically different products under the same reaction condition
- If the starting materials are diastereomers, their products will also be diastereomers of each other
E1 reactions are not stereospecific, so stereoisomeric starting materials will not give stereoisomerically different products
- Since the hydrogen does not have to be anti-periplanar to the leaving group, the two other groups attached to the β-carbon are free to switch places through bond rotation (as long as the β-hydrogen is still coplanar with the empty p-orbital)
- The product will be a mixture of diastereomers
- For starting materials that are diastereomers of each other, their product will yield the same mixture
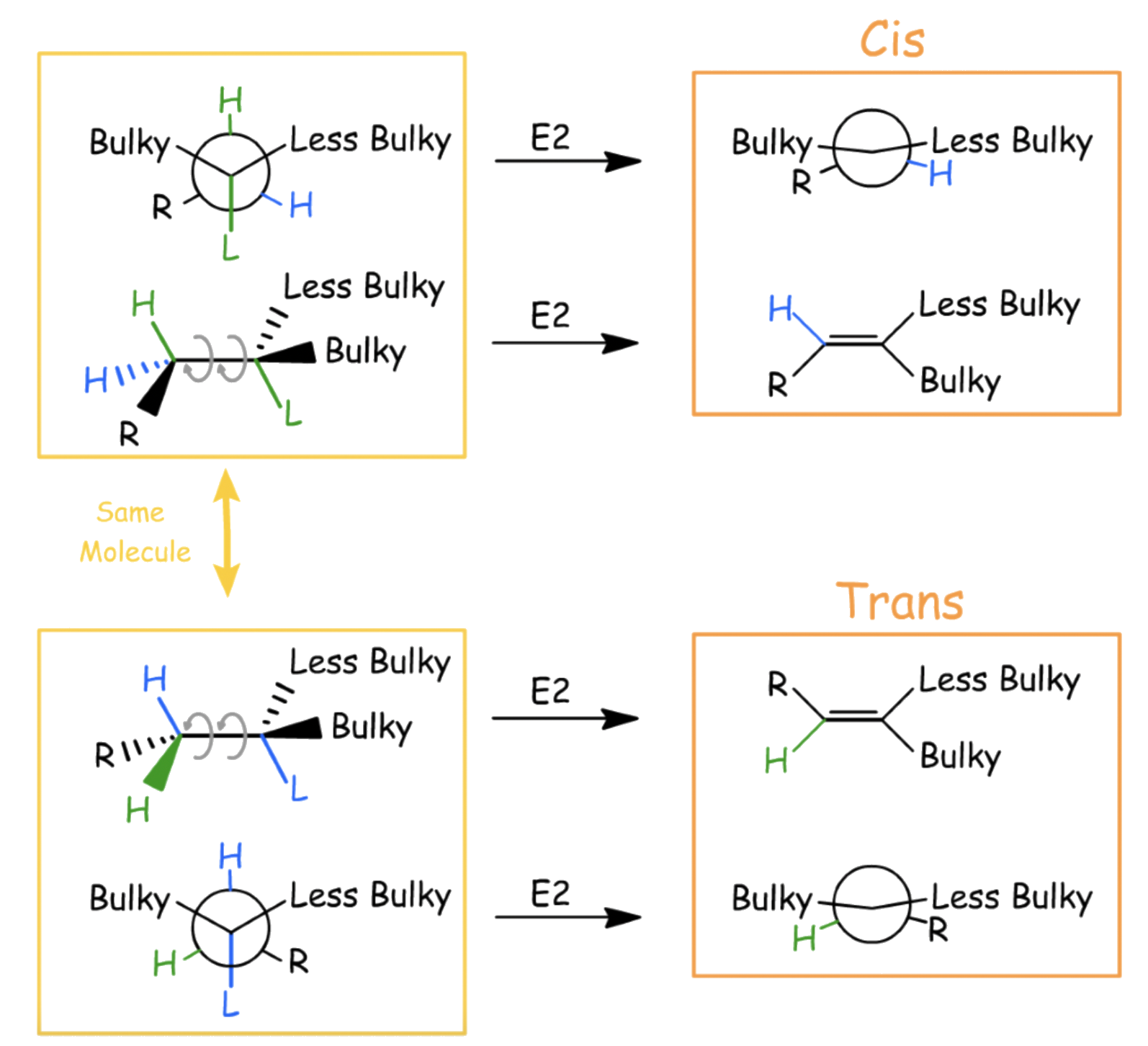
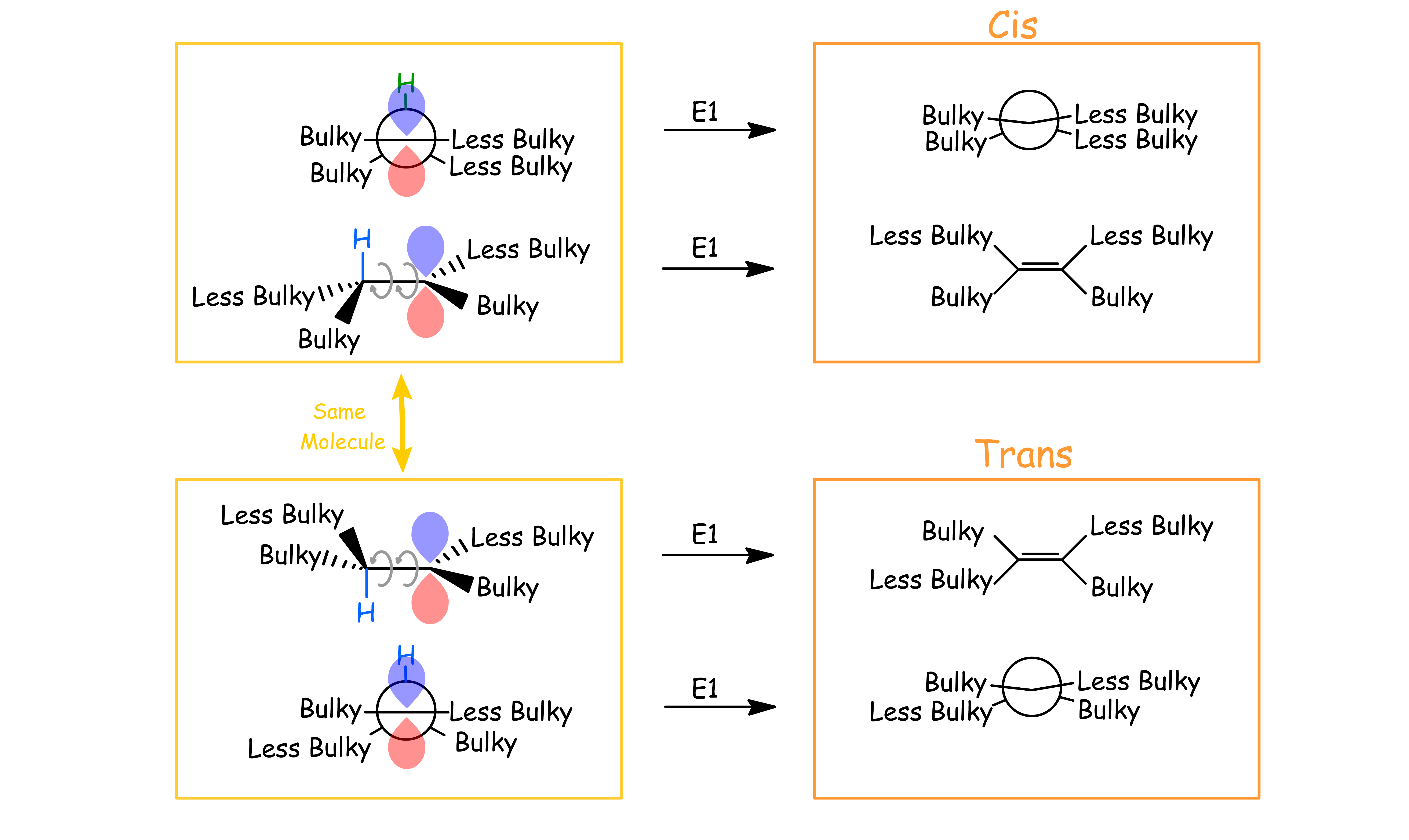
Both E1 and E2 reactions are stereoselective and favors the formation of the trans-isomer when there are two β-hydrogens
For E2 reactions, if there are two β-hydrogen present, the product will favor the formation of the trans isomer
To form the cis-isomer, the alkane must first adopt a conformation where the substituent on the β-carbon is synclinal with the bulky group
- This conformation increases the steric hinderance of the molecule
- Formation of cis-isomer is not favored
To form the trans-isomer, the alkane must first adopt a conformation where the substituent on the β-carbon is anti-periplanar with the bulky group
- This conformation decreases the steric hinderance of the molecule
- Formation of trans-isomer is favored
For E1 reactions, the product will favor the formation of the trans isomer ( even in the absence of an additional β-hydrogen )
- The two β hydrogens can are free to switch places through bond rotation (as long as the β-hydrogen is still coplanar with the empty p-orbital)
¶ Preference between E1 and E2 due to the structure of the Electrophile
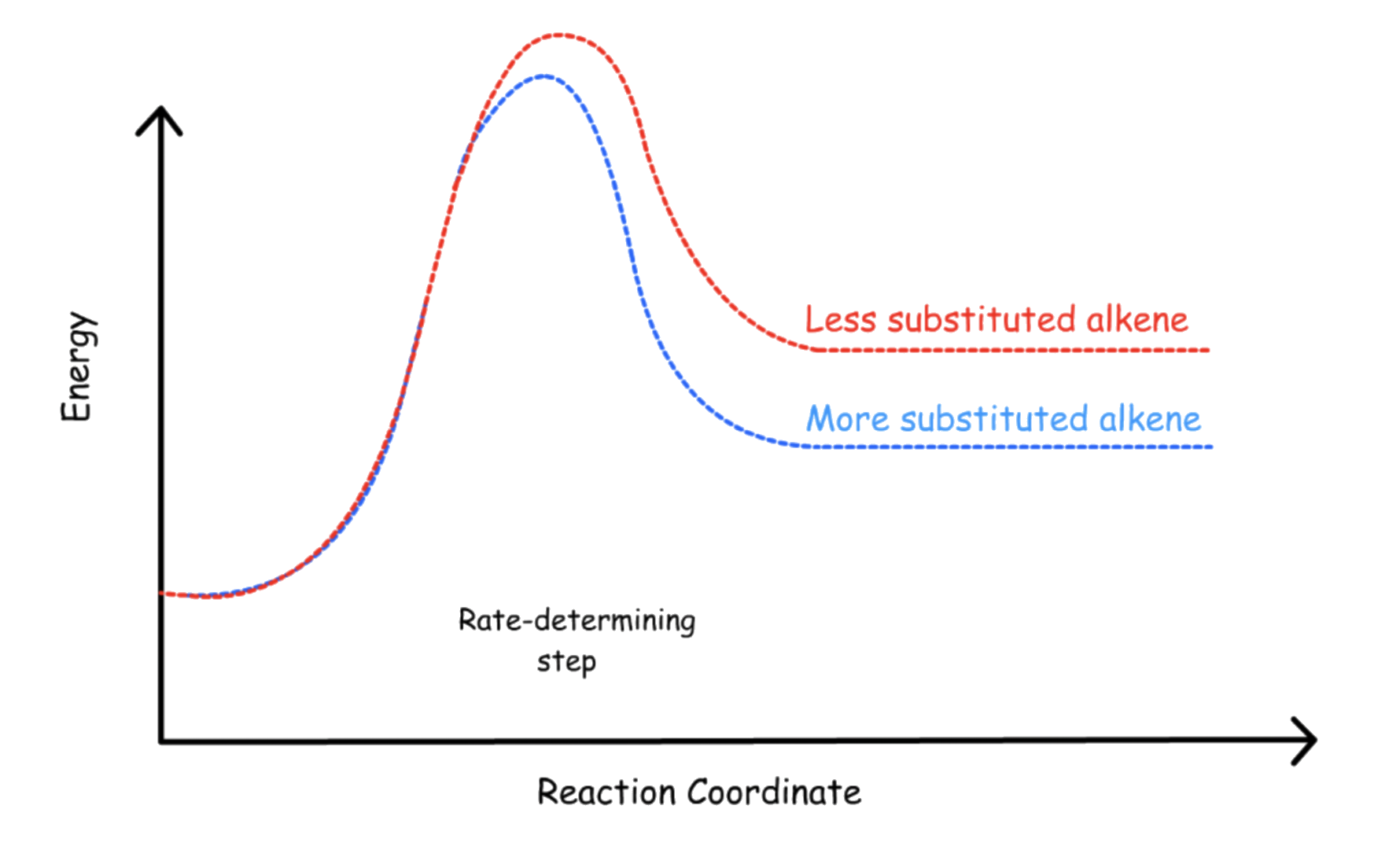
E1 reaction involves a carbocation intermediate. According to the Hammond's Postulate, anything that can stabilize the carbocation can stabilize the transition state and thus increase the rate of reaction
- Carbocations with a higher degree of substitution ( of electron-donating groups ) will be stabilized to a greater degree
- Hence, the rate of reaction increases when the degree of substitution of the α carbon increases
- The formation of the carbocation intermediate is the rate-determining step
For E2 reactions, according to the Hammond's Postulate, anything that can stabilize the alkene ( product ) can stabilize the transition state of the rate-determining step ( the only step )
- Alkenes with a higher degree of substitution will be stabilized to a greater degree and E2 reaction is favored
- Hence, the rate of reaction increases when the degree of substitution of either the α or β ( or both ) carbons increases
- The effect is greater when the degree of substitution of the beta-carbon increases as it also increases the number of β-hydrogen available ( thus increasing the probability of reaction )
¶ Preference between E1 and E2 due to Base
In E1 reactions, the base does not affect the rate of reaction
- Carbocation is highly unstable, so it will react with strong and weak bases equally fast
In E2 reactions, both the concentration and the type of base is important
- Since the base needs to directly to pull a proton off the carbon chain, it has to be a strong base
- The reaction is first order with respect to the concentration of base
¶ Controlling SN1, SN2 & E1, E2 reaction
SN1, SN2 & E1, E2 reactions take place at sp3 hybridized carbons
- SN1 & SN2 reactions are nucleophilic aliphatic substitution reactions
- E1 & E2 reactions are elimination reactions
The mechanism, choice of reagents and reacting conditions of the substitution reaction and the eliminations are very similar
- Both SN1 and E1 reactions involve the formation of a carbocation intermediate
- Both SN2 and E2 reactions are concerted one step mechanism
All four reactions will often compete with each other, which one prevails will depend on the reacting conditions
- Both the substitution and elimination reaction requires similar reacting conditions and reagents
For substitution reaction, a nucleophile is required. For elimination reaction, a base is required. Since nucleophiles can also act as base and vice versa, the two reactions will often occur simultaneously
- We can control which reaction will occur predominately by adjusting the reaction condition
¶ Leaving Groups
Both substitution and elimination reaction requires a good leaving group to occur
- The rate of reaction depends on the leaving group ability
There are two factors that affect the leaving group ability
1. The strength of the bond between carbon and the leaving group
- The stronger the bond / the higher the bond enthalpy, the less likely the leaving group is going to be ejected
2. The stability of the leaving group
- The more stable the leaving group is with the electron pairs in the solvent, the more likely the leaving group is going to be ejected
- The stability of the leaving group is related to its pKaH in that particular solvent: Lower = More stable
The leaving group ability of certain functional groups are too low to participate in nucleophilic substitution reaction, hence, the functional group must be converted to a better leaving group
¶ Solvation effects
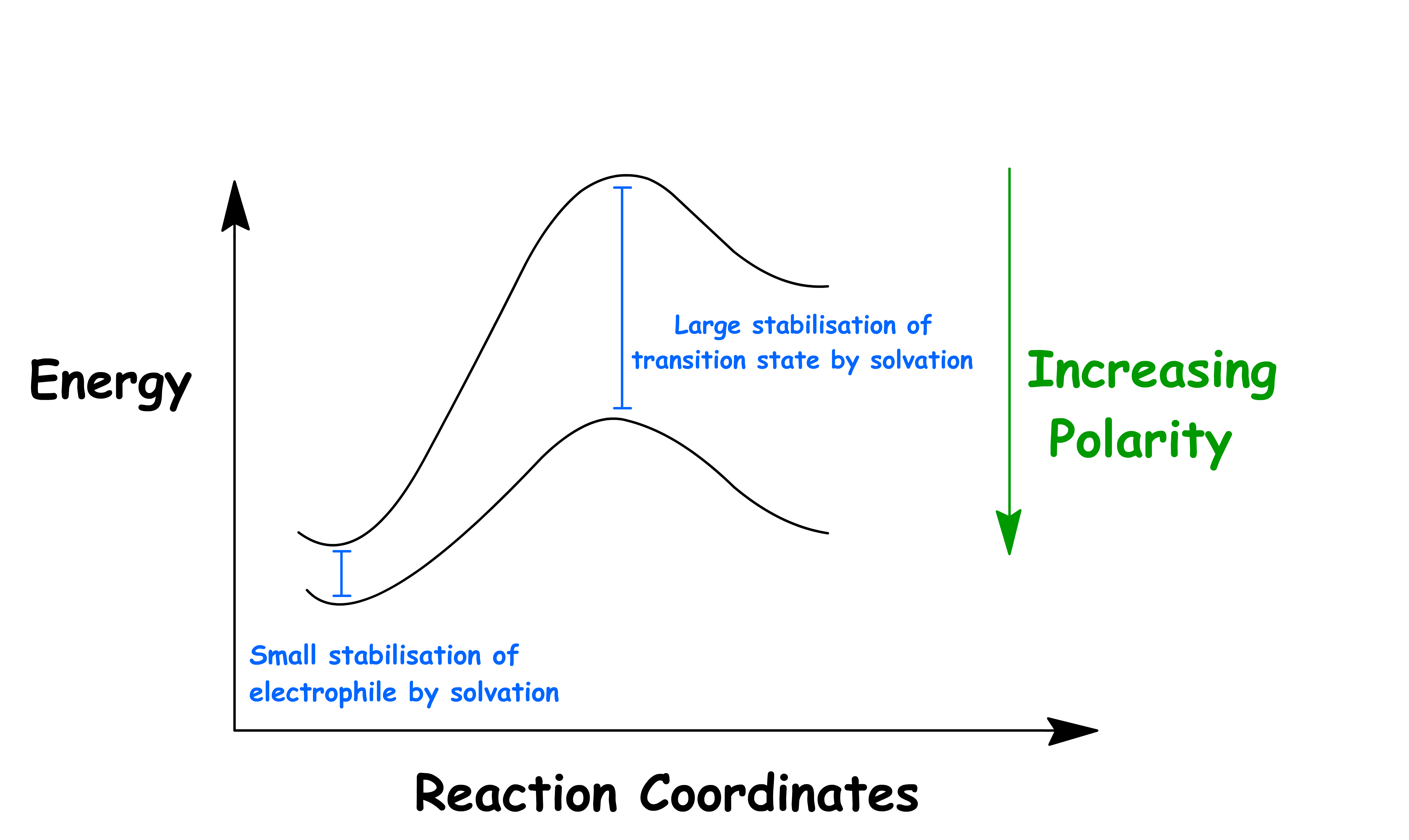
The transition state of both SN1 and E1 reactions is similar to a carbocation loosely bonded to the leaving group. Hence, the activation energy can be lowered by stabilizing the carbocation and the leaving group
- The carbocation is stabilized by sigma conjugation, while the leaving group is stabilized through solvation
- The leaving group is an anion, so it can be stabilized by polar protic solvents
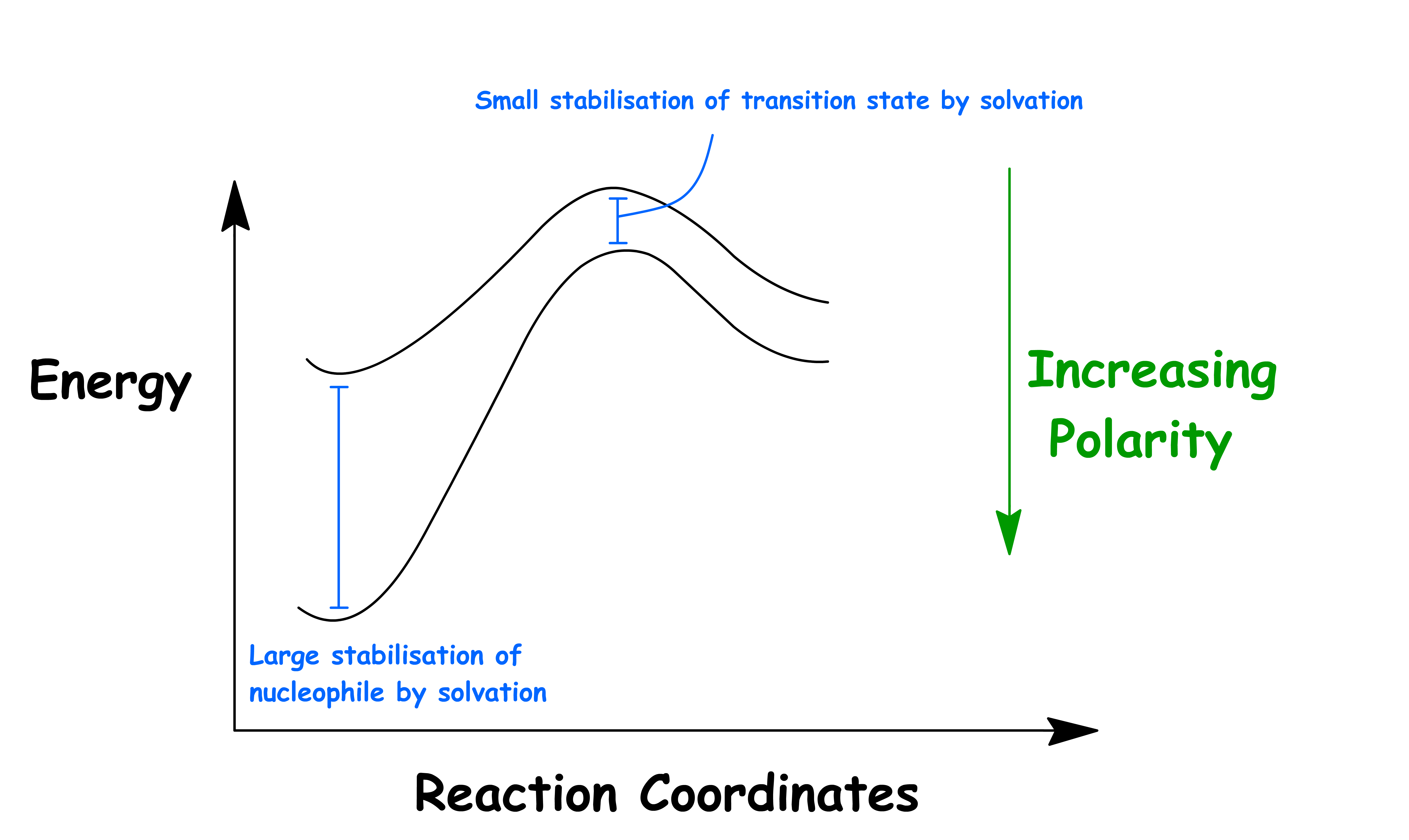
The negative charge of the transition state of both the SN2 and E2 reactions is relatively dispersed, so polar solvents can only stabilize it to a small degree
When using polar aprotic solvents, since the anion is weakly solvated, and there is little cation-anion association in polar aprotic solvent, the term "naked anion" is used to describe the state of anions in these solvents
- No solvent shell
Since substitution reactions requires nucleophiles, while elimination reaction requires bases, protic solvent usually favors elimination reaction
- The protic solvent molecules cling to the nucleophile like a protective coating, thus decreasing their nucleophilicity
¶ Steric Effects
In both SN1 and SN2 reactions, the nucleophiles need to interact with the carbon in close proximity
- Especially SN2 reactions
- Substitution reactions are relatively sensitive to steric hinderance
In both E1 and E2 reactions, the base only need to pick up the β-hydrogen
- Since the β-hydrogen is often far apart from the carbon chain, the base will not suffer from a strong steric effect induced by the carbon chain
- Elimination reactions tend to be less sensitive to steric hinderance
A bulky electron-donor will act as a base rather than a nucleophile, thus favoring the elimination reaction
- A bulky base also means that the product of the elimination will obey the Hofmann's rule instead of the Saytzev Rule
¶ Nucleophile or Base
Whether SN1 or SN2 will occur depends on the hardness and softness of the nucleophile. Whether E1 or E2 reactions will occur depends on the strength of the base
- Soft nucleophile favors SN2 reactions
Strong base favors E2 reactions
- SN1 and E1 reaction will proceed with any nucleophile or base. However, their rate of reaction is rather slow, so they will often be out competed by SN2 or E2 reactions when the nucleophile is soft or when the base is strong
¶ Temperature
The change in entropy of a substitution reaction is close to 0, since the number of molecules on the reactant side is the same as that of the product side
- = small
There is a larger change in entropy for elimination reactions, since the number of molecules on the product side is greater than that of the reactant side
- = large
For substitution reactions, the change in Gibbs Free Energy is basically the change in enthalpy, so changing the temperature will not have a significant effect on the equilibrium
- Chaning the temperature barely affect the degree of substitution
For elimination reactions, the change in Gibbs Free Energy will be amplified by changing the temperature, since a change in temperature amplifies the entropy term
- The Gibbs Free Energy becomes more negative when the temperature increases
- Elimination reaction is favored at high temperature