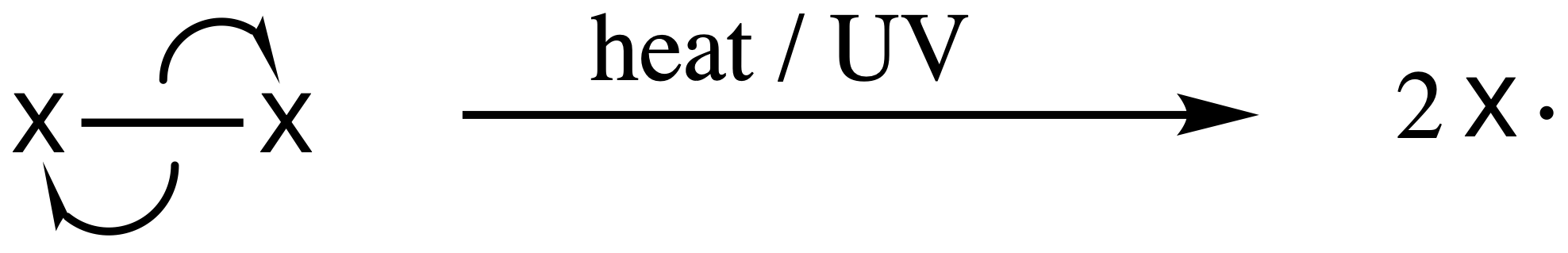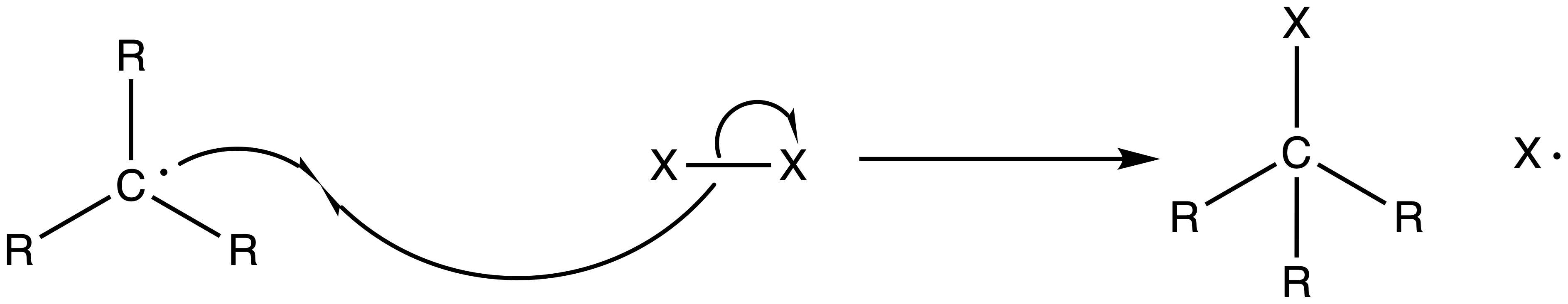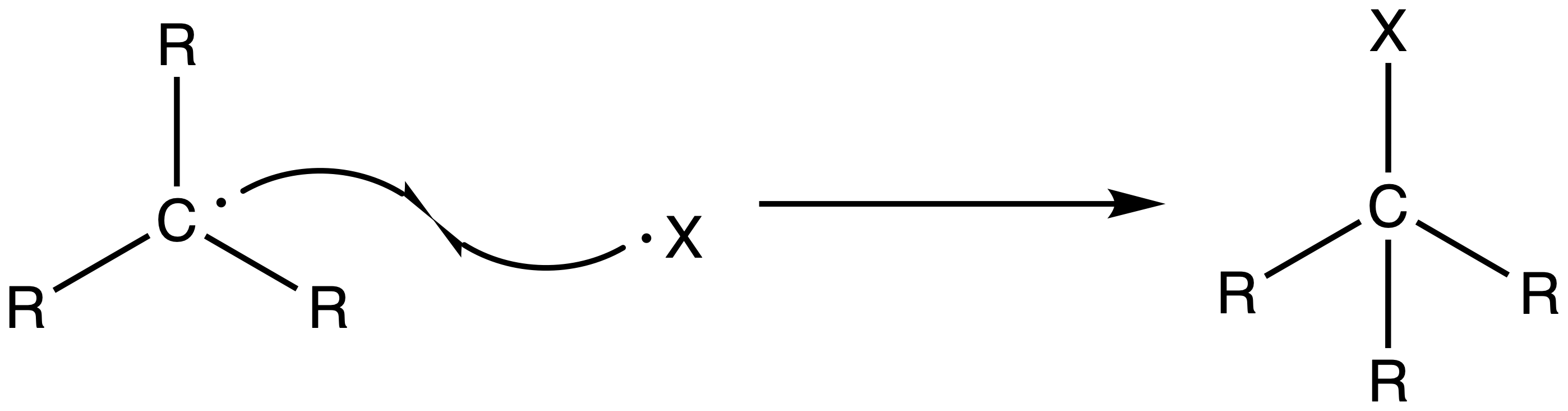¶ Mechanism
1. Chain Initiation Step
- Under the irradiation of an ionizing radiation or high temperature, the halogen undergoes homolysis to give two radicals
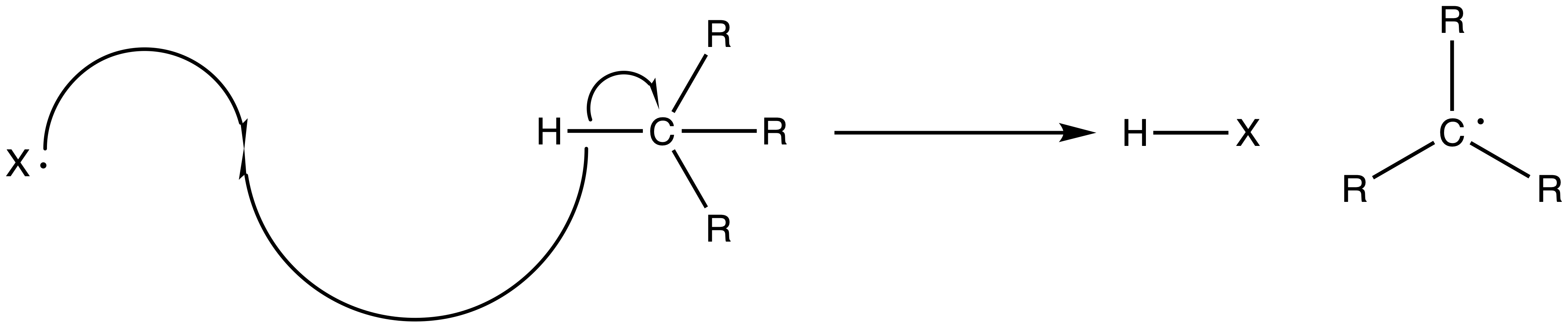
2. Chain Propagation Step
- The halide radical abstracts a hydrogen from a neighboring alkane to form HCl and a radical carbon chain
- The alkane radical can then react with other halogen to reform the halogen radical
- The radicals can also react with its own species such that the products are identical to the starting materials. We call these non-productive steps
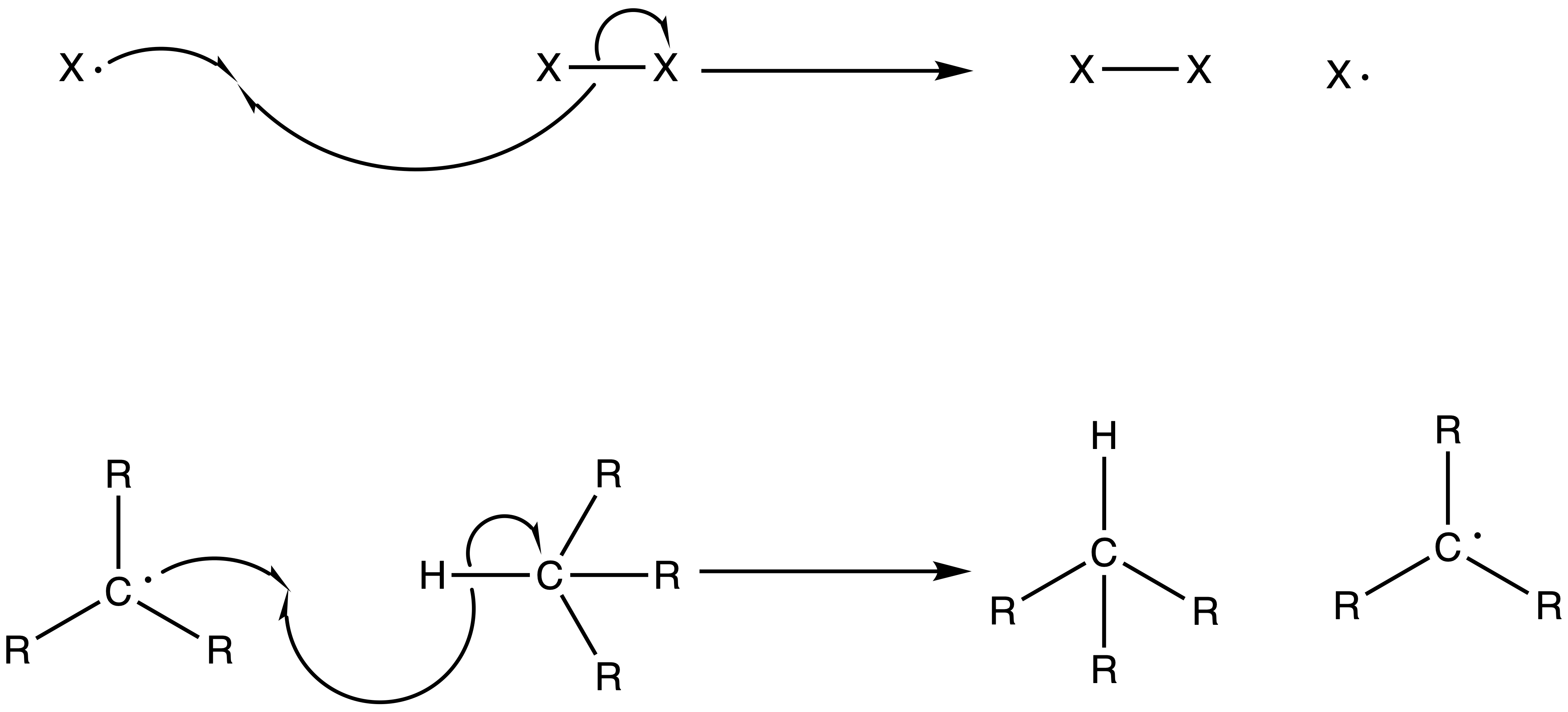
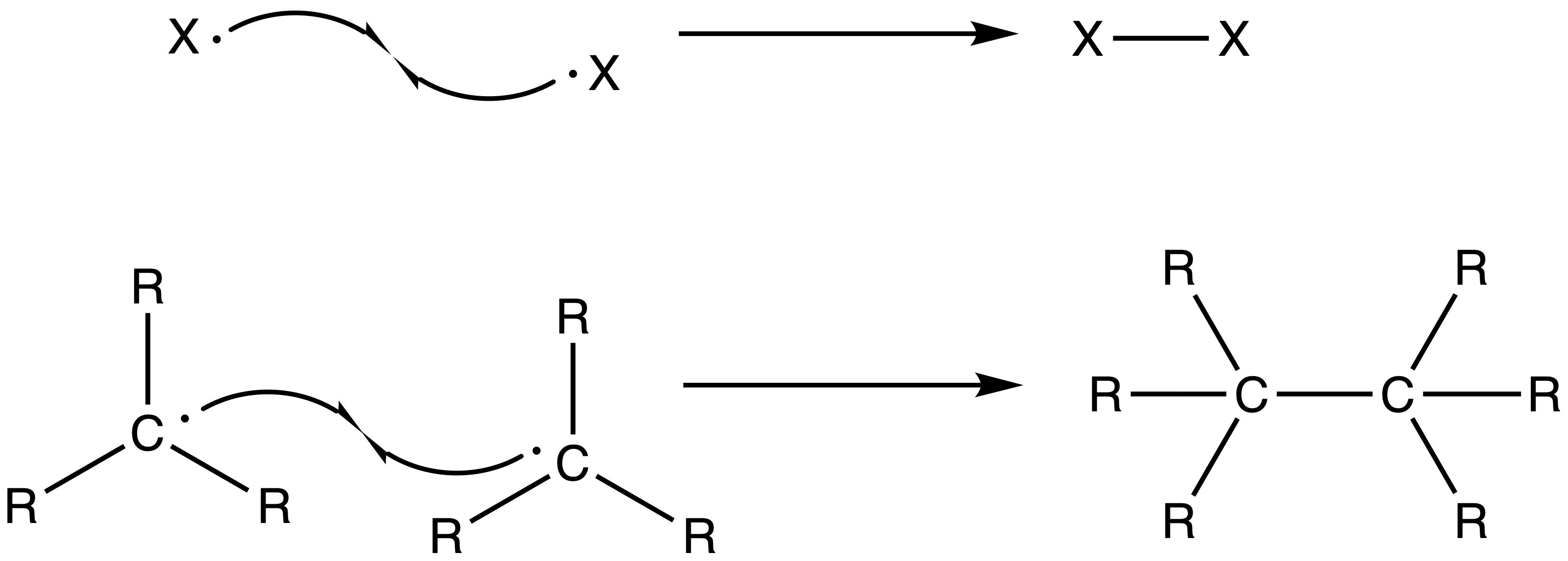
3. Chain Termination Step
- Two radicals recombine, thus terminating the chain reaction
- The radicals does not necessarily recombine to form the product
¶ Effects of different Halides
The bond strengths of halogens are relatively weak, so they are able to undergo homolytic cleavage relatively easily
- As we travel down the group, the size of their valence orbitals become larger and more diffuse, so the bond enthalpy decreases
Despite the increasing ease for homolysis, the rate of reaction does not increases down the group. Quite the contrary, the rate of reaction decreases down the group
- Fluorine reacts explosively
- Chlorine reacts violently
- Bromine reacts smoothly
- Iodine barely reacts at all
The reason why the rate of reaction has such a trend has a lot to do with the overall enthalpy of the reaction
- For the initiation step, generation of the halogen free radical, uses the radiation or heat to break the X−X bond, so no additional energy is required for this step
- For the propagation step, The enthalpy of the dissociation of C–H should be the same for all halogens. However, the bond enthalpy of formation of X–H is different for each halogen. The bond enthalpy of X – H decreases down the group
- For fluorine and chlorine, the overall enthalpy of the propagation step is negative, so the step is exothermic
- For bromine and iodine, the overall enthalpy of the propagation step is positive, so the step is endothermic
- For the termination step, we only need to calculate the enthalpy of formation of the alkyl halide
The bond enthalpy of X – H decreases down the group
- When we account for the overall enthalpy of the reaction ( propagation and termination ), we can see that the overall enthalpy decreases down the group
- Fluorine, chlorine, bromine all have an overall negative enthalpy, so their reactions are favorable
- Iodine have an extremely low overall enthalpy, and may even be positive depending on the alkane chosen, so the halogenation of iodine through radical substitution is unfavored
¶ Regioselectivity
Free radical substitution is regioselective
- There are two factors that affect the regioselectivity
1. The number of equivalent hydrogen
- Statistically speaking, the higher the proportion of equivalent hydrogens, the more likely it can be substituted
- This is a kinetic effect
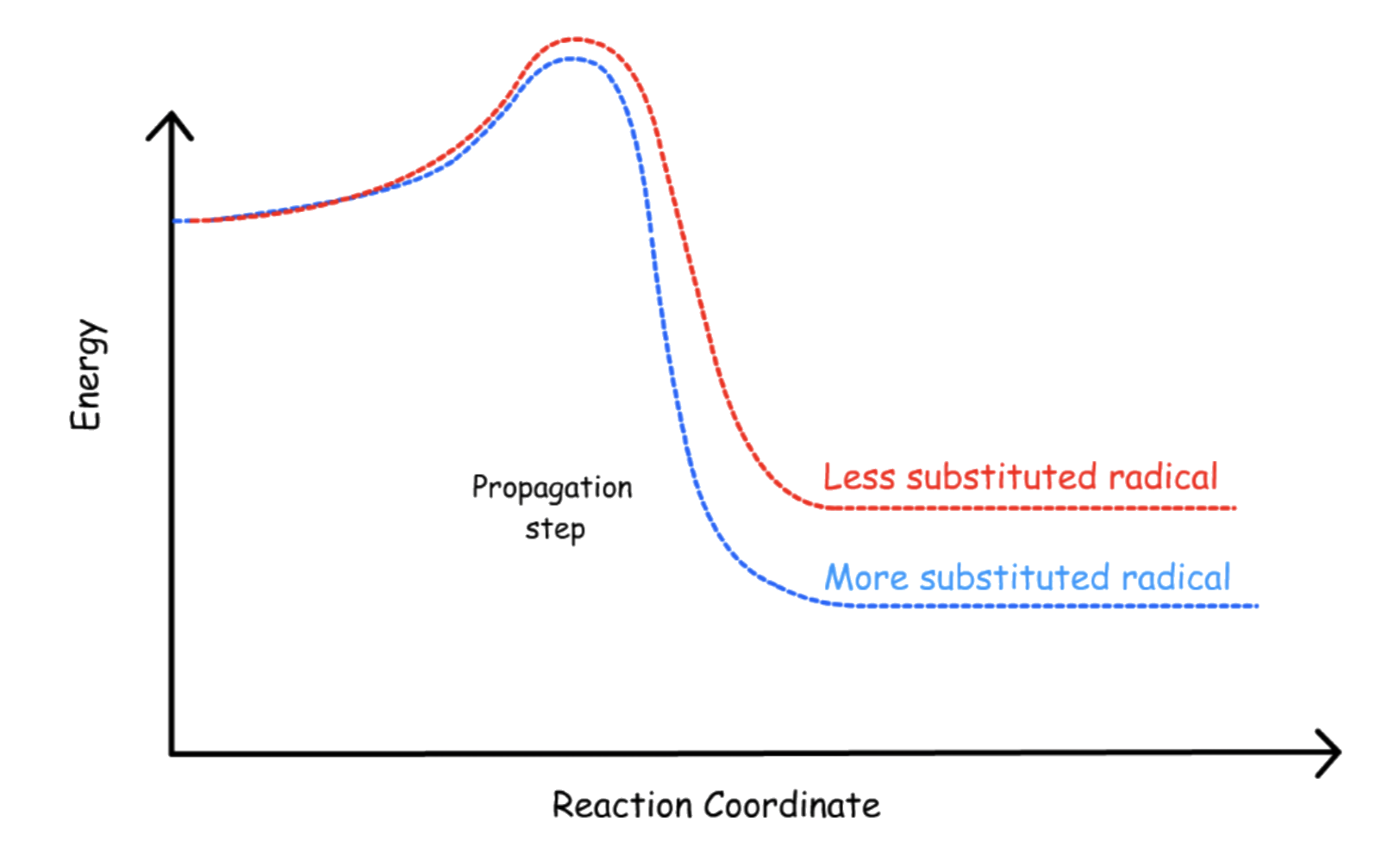
The degree of substitution of the radical
- As established before, radicals with a higher degree of substitution are stabilized to a greater extent
- According to Hammond's Postulate, the stabilization of the reactant / product can also stabilize the transition state, thus lowering the activation energy
The effect of the degree of substitution of the radical depends on which halogen is used
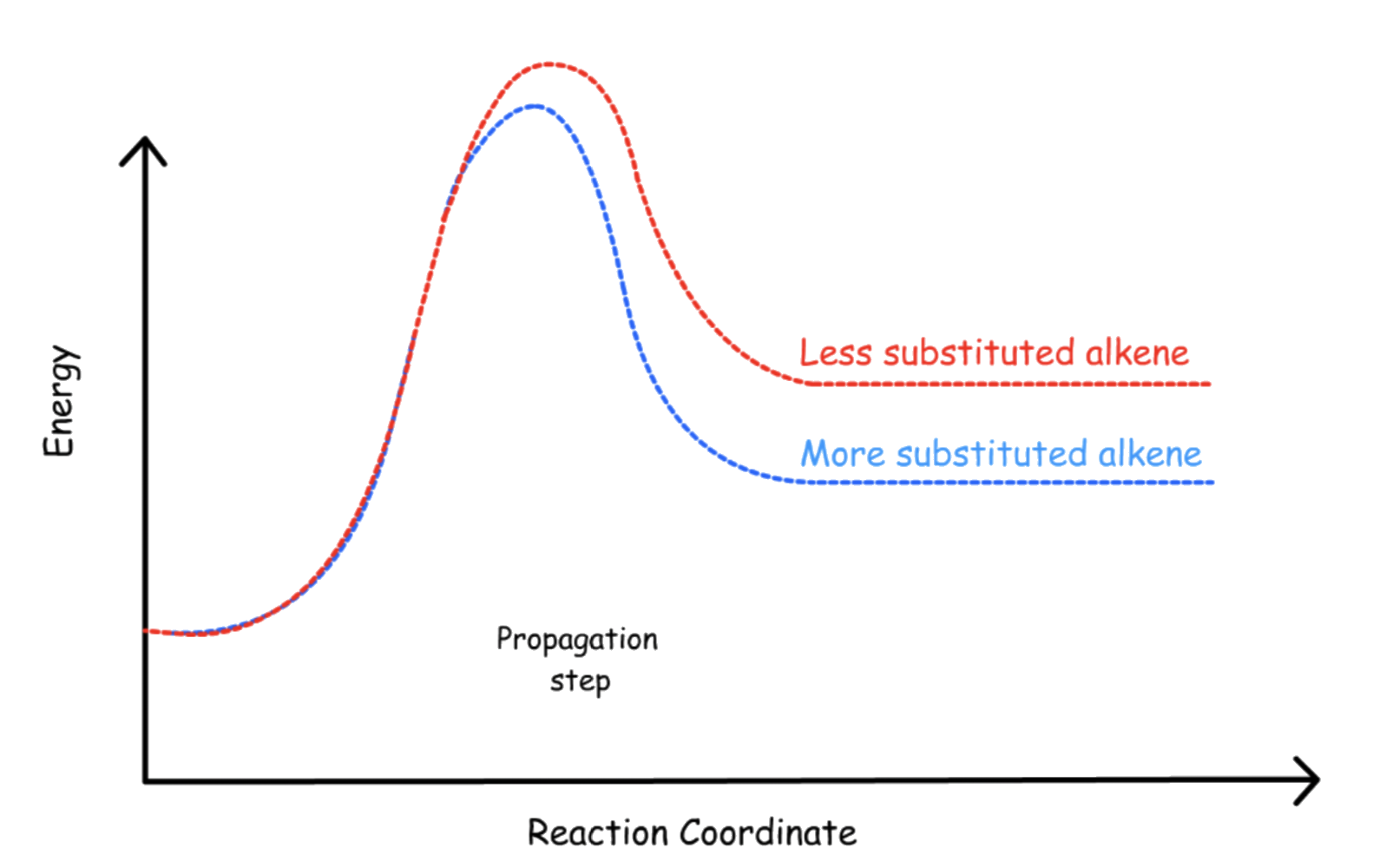
The propagation step is the rate-determining step, so anything that can stabilize the transition state of said step will be favored
- For fluorine and chlorine, their propagation step is exergonic, so the transition state resembles the reactant more than it does the intermediate. Hence, the stabilization given by a higher degree of substitution is insignificant for fluorine and chlorine
- For bromine and iodine, their propagation step is endergonic, so the transition state resembles the intermediate more than it does the reactant.Hence, the stabilization given by a higher degree of substitution is significant for bromine and iodine