¶ Premise of Spectroscopy
Spectroscopy concerns itself with the interaction of matter and light
-There are different types of electromagnetic radiation, and each one can interact with matter in different waysDue to the quantized nature of matters, only certain frequency of radiations can be absorbed or emitted, which leads to a lot of interesting behavior
- The principles of spectroscopy can be applied in analytical chemistry
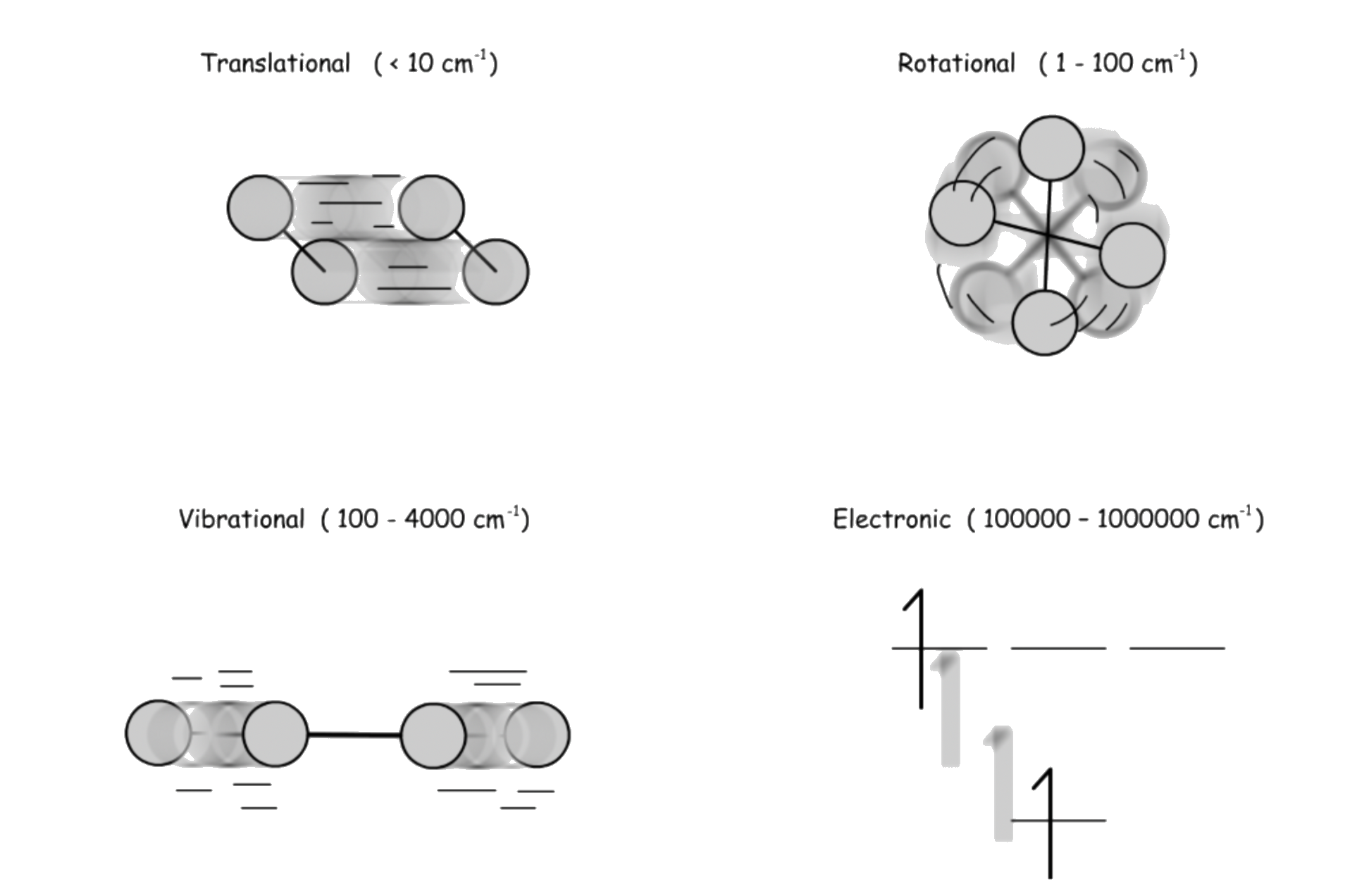
¶ Types of Molecular Energies
Molecular energies are quantized
- For translational energy, it is basically continuous
For the promotion and demotion of electron, is used instead of wavenumber
- To convert between Joule, electron Volt and wavenumber, the following formulas can be used:
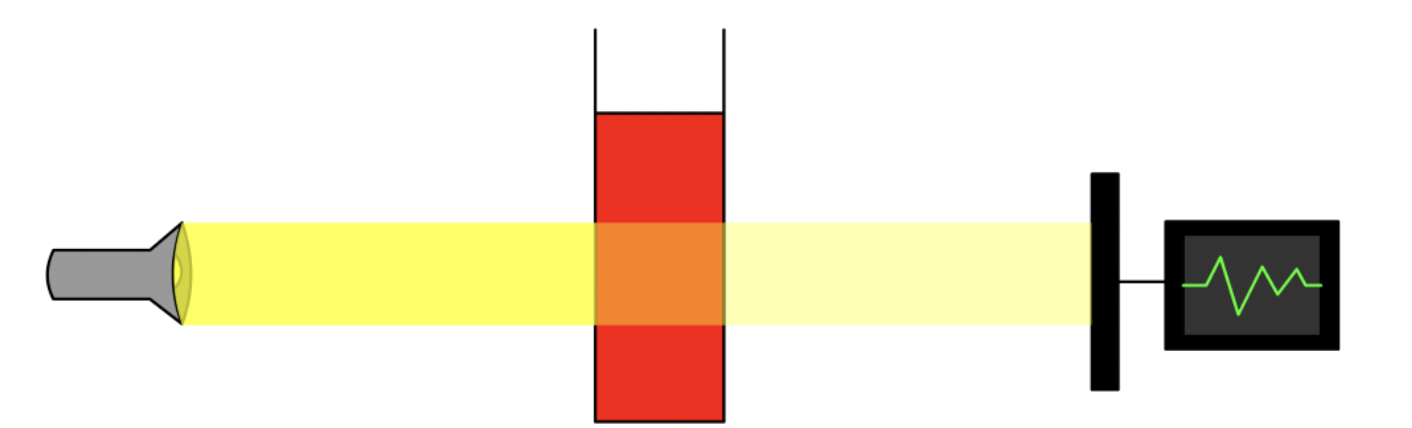
¶ Basic Principle of Spectroscopy
Spectroscopy revolves around two key concepts:
- The absorption/ emission of light
- The quantization of energy
When light passes through the sample, part of it will be absorbed
- The absorption pattern differs for different molecules
Different type of light will be used in different spectroscopy
- NMR: Radio radiation
- Rotational: Microwave radiation
- Vibrational: Infrared radiation
- Electronic: Visible light & Ultra Violet radiation
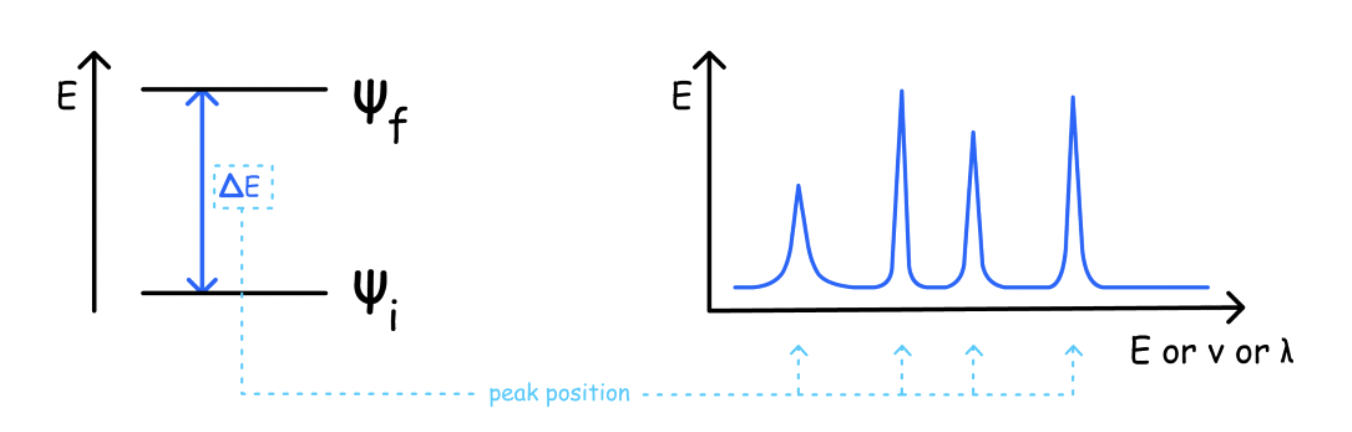
The spctra are obtained by finding the absorption energies and intenisties of light
The intensity of a peak is determined by several factor:
- Transition dipole ( probabilty of transition )
- The amount of sample
- Population of initial energy state
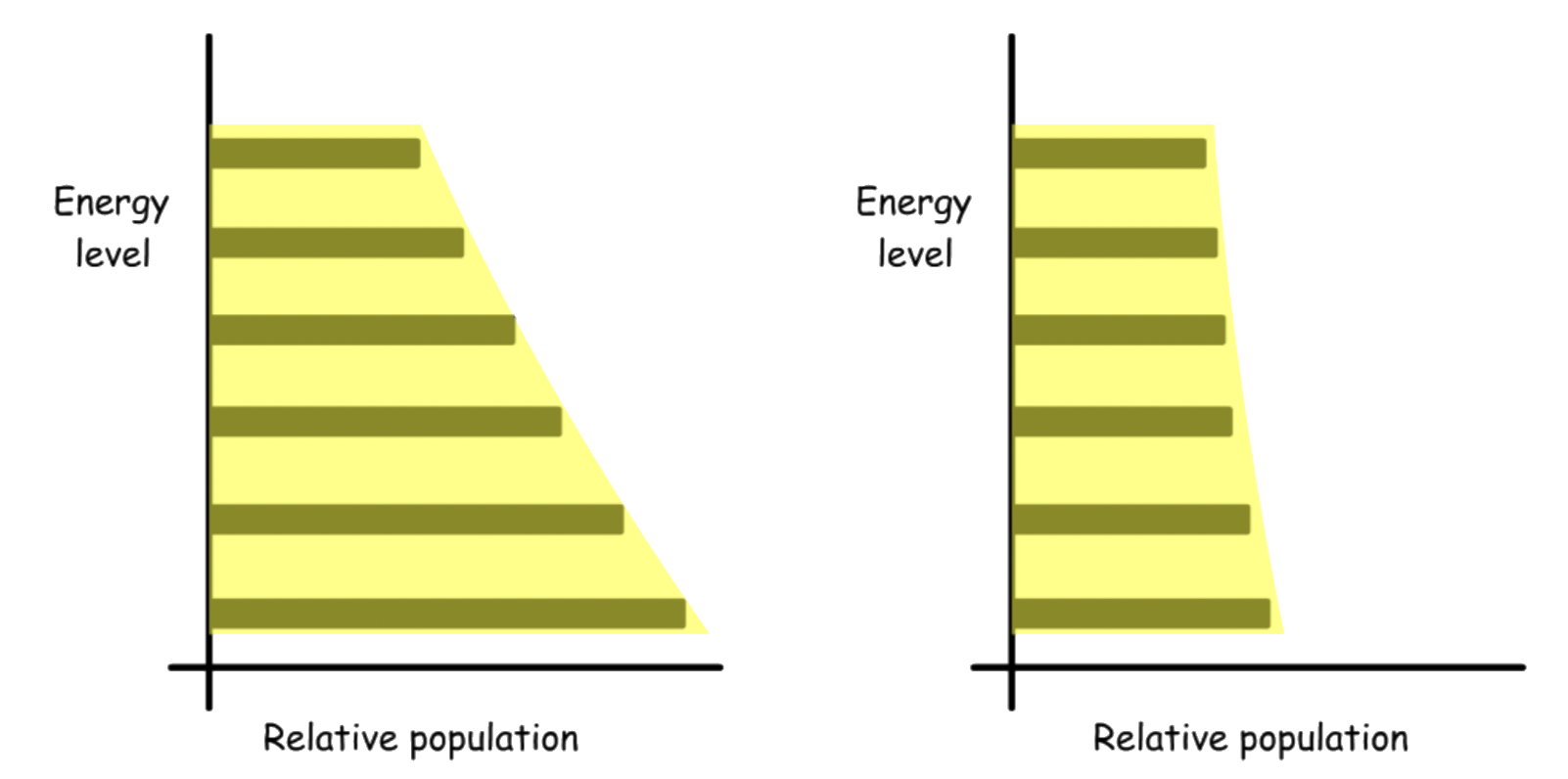
¶ Population of states
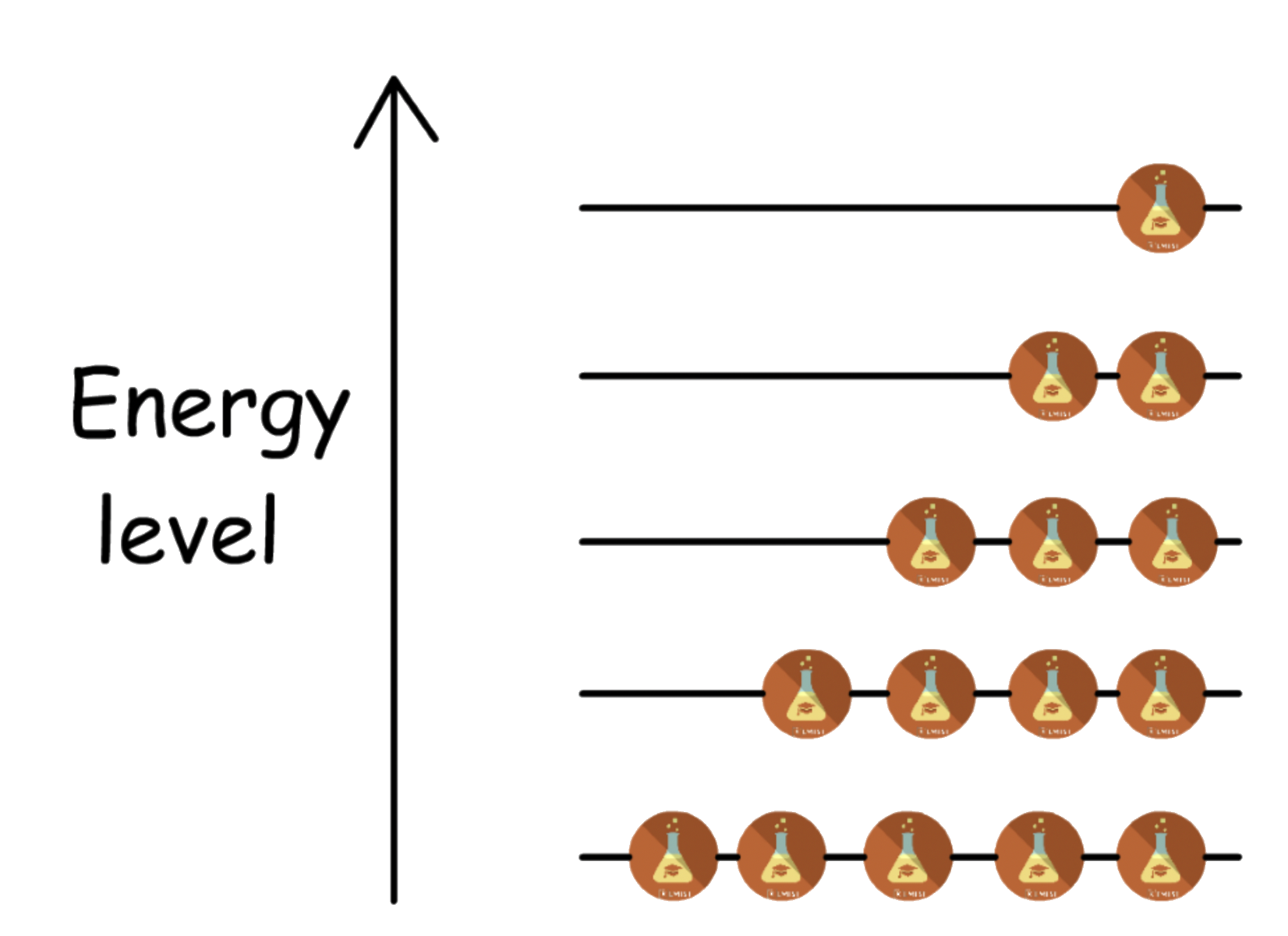
The ratio of the population of states can be described by the Boltzmann distribution:
At any given moment, the population of states cannot all be in the ground state unless the temperature is 0 K.
- The continuous thermal agitation that molecules experience at temperature above 0 K ensures the molecules are distributed over all possible energy states
According to the Boltzmann distribution, If there are no degenerate states, the population of the higher energy level will always be smaller than that of the lower energy level
-At high temperature the disparity is less apparent than that of low temperature
- The ground state will have the highest population
When degeneracy is taken into account, the relative population may not necessarily peak at the ground state
- When the difference in energy is 0 ( degenerate states ), the ratio of population is 1
- The lower the ratio of population of the two state, the more intense the peak will be
¶ Transition Dipole Moment
The transition dipole moment or transition moment is the electric dipole moment associated with the transition between the two states
Transition strengths are used to describe transition probability
- Transition is forbidden when the transition dipole moment is 0
Interaction with electromagnetic radiation results in a transition dipole moment ( )
The probability of transition from one energy level to another depends on two things
- The nature of initial and final state wavefunctions
- How strongly photons interact with an eigenstate
The general formula of transition dipole moment is given by the integral :
is the dipole operator and can take different forms in different context
- e.g. permanent dipole, change in dipole moment, induced dipole moment
A dipole operator is necessary because light is an oscillating electric field, so only objects with some sort of charge can interact with light
- Everything possess a charge in some ways, because protons and electrons possess charges
- The type of charge ( dipole ) the object can have will determine whether said object can interact with a certain range of wavelength of light
- The term "dipole" is being used loosely here
The dipole operator, , whether it is permanent dipole, change in dipole moment or induced dipole moment, varies linearly with respect to some sort of distance, r
- In otherwords, is an odd function
- To ensure that the transition dipole moment is not zero, the overall function should be an even function
- The integral of an odd function over symmetric limits returns a value of zero, while for an even function this is not necessarily the case
- Since is an odd function, the product of the other two function should be odd
- One of the wavefunctions should be an odd function, while the other should be an even function